Author: Sireedhorn Ann Assavanop, MD 1, Tamiris Soares, MD 2, Marina F. Englesakis, BA(Hons), MLIS 3, Yasmine Hoydonckx, MD, MSc, FIPP 4
Author Affiliation:
1 Department of Anesthesia and Pain Management, University of Toronto; University Health Network-Toronto Western Hospital and Women’s College Hospital, Toronto, Ontario, Canada
2 Department of Anesthesia and Pain Management, University of Toronto; University Health Network-Toronto Western Hospital and Women’s College Hospital, Toronto, Ontario, Canada
3 Library & Information Services, University Health Network, Toronto, Ontario, Canada
4 Department of Anesthesia and Pain Management, University of Toronto; University Health Network-Toronto Western Hospital and Women’s College Hospital, Toronto, Ontario, Canada
Competing Interests: The author/s declare no competing interests.
Issue: 12.01
DOI: 10.30756/ahmj.2024.12.01
Received: May 13, 2024
Accepted: Jun 11, 2024
Published: Jun 19, 2024
Recommended Citation: Assavanop SA, Soares T, Englesakis, MF, Hoydonckx, Y. Interventional Management Of Neuropathic Ocular Pain – A Scoping Review. Ann Head Med. 2024;12:01. DOI: 10.30756/ahmj.2024.12.01
Neuropathic Ocular Pain (NOP) is a debilitating and refractory pain condition. This scoping review is the first to summarize the current evidence of efficacy of interventional treatment options for NOP. Databases were searched for studies published up to March 31, 2023. Two reviewers screened and extracted data, and performed the risk of bias analysis. Twelve studies were included, consisting of 4 cohort studies and 8 case series/reports, with a total of 87 patients. Eight interventions were defined: stellate ganglion block (n=1), trigeminal nerve blocks (n=3), retrobulbar block (n=1), pulsed radiofrequency of sphenopalatine ganglion (n=1), Onabotulinum-Toxin A(n=1), trigeminal nerve stimulation (n=1), intrathecal drug delivery (n=1) and transcutaneous electrical trigeminal nerve stimulation (n=3). Procedures were found to be safe and demonstrated analgesic effect. Follow-up ranged from 24h to 12 months. Substantial heterogeneity across studies was found, and quality was deemed low and of moderate risk of bias. High-quality studies are urgently needed.
Introduction
The cornea is one of the most densely innervated tissues in the body.1 Ocular surface pain is a condition that is characterized by discomfort, irritation, or burning sensation in the eyes. This condition was usually grouped under the umbrella term “dry eye (DE)”, but recent research has shown that it can occur independently of tear dysfunction. 2 The prevalence of ocular surface pain varies depending on the definition of symptoms and the studied population, ranging from 5% to 50%. 3, 4 This is a complicated and multifaceted condition that is linked to multiple risk factors, which can significantly disrupt an individual’s daily life both physically and mentally, resulting in a poor quality of life. 5-7 Ocular surface pain can be classified into nociceptive and neuropathic pain based on their respective causes and presentations. This review primarily focused on a discussion of Neuropathic Ocular Pain (NOP), also known as Corneal Neuralgia, Keratoneuralgia, or Burning Eye Syndrome. 8
NOP can be further classified based on the location of the nerve lesion within the somatosensory system: “peripheral”, which is characterized by dysfunction of corneal sensory nerves and/or periocular nerve fibers; “central”, involving dysfunction in ascending and descending central nervous system (CNS) fibers, and “autonomic”, which affects the autonomic nervous system (ANS). 9 The etiologies of NOP include ocular diseases (dry eye disease,5, 10, 11 herpetic keratitis, 12 recurrent erosion syndrome 8), post-traumatic (radiation keratopathy 2, post-chemotherapy 13, trauma 11, post-refractive surgery 14, 15), systemic diseases (Sjögren’s syndrome, lupus) 11, and psychological comorbidities (anxiety, depression, and history of posttraumatic stress disorders). 16, 17
The symptoms of NOP can vary and may include aching, burning, foreign body-like, dryness, irritation, discomfort, squeezing, pressure, itchy, light sensitivity, allodynia, and hyperalgesia. 18 Some patients may also experience periocular pain, facial pain, migraine headaches, and hyperacusis. Visual disturbances have also been reported.
Several pharmacological and non-pharmacological options for NOP have been investigated, including antidepressants and anticonvulsants. However, a significant proportion of patients remain refractory to treatments. 8, 9, 17 Several interventional (percutaneous) procedures have been successfully used in the treatment of complex chronic pain states such as complex regional pain syndrome and neuropathic pain 19-23, but their therapeutic role for NOP have not been completely established. Therefore, the objective of this scoping review is to evaluate the efficacy of these interventional options for the treatment of NOP.
Methods
This scoping review was performed according to the Arksey and O’Malley’s framework for conducting a scoping review, with modifications proposed by Levac et al. We specified the research questions, identified the relevant literature, selected the studies, mapped the data, and synthesized the data to report the results.
Search Strategies And Terms
We conducted a comprehensive search of the literature from database inception to March 31, 2023, with the assistance of a medical information specialist (M.E.). The following databases were searched: MEDLINE, 1946 onward; MEDLINE Epub Ahead of Print and In-Process, In-Data-Review & Other Non-Indexed Citations; Embase Classic/ Embase, 1947 onward; Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Web of Science, and Scopus. The search was restricted to human subjects. We searched for randomized and nonrandomized trials, case series and case reports, systematic reviews and meta-analyses by using combinations of subject headings and keyword terms for “eye or ocular” and “neuropathic pain” and “interventions”. Details of the search strategies are provided in Supplement 1, and a summary of the search history record is presented in Supplement 2.
Eligibility Criteria
A population, concept, and context (PCC) approach was followed for this scoping review. 24, 25
Population
Studies included in the clinical analysis focused on adult patients (age 18 years and older) who suffered from NOP.
Concept
The concept of interest was the role of (percutaneous) pain interventions in the treatment of NOP.
Context
The context of interest was to assess the efficacy in terms of change in pain intensity, improvement of functional and psychological outcomes, and quality of life. Sustainability of analgesic benefit and adverse effects were also noted.
Study Selection Process
All citations were independently screened on title and abstract for eligibility by two reviewers (S.A.A. and T.S.) as per the inclusion criteria. Covidence® was used as a management tool. Papers of interest were then full text screened. Data was independently extracted by two reviewers (S.A.A. and T.S.). Any disagreement was resolved through discussion with senior author (Y.H.).
Data extraction
Extracted data included number of patients, type of study, patient characteristics, details of pain condition, details of interventions and comparators (type of injectate, dose, guidance technique), follow up time points, outcomes, and adverse effects of the interventions. The data was entered into prespecified tables on a standardized data extraction form. The data collection form was pilot-tested before its use.
Assessment Of The Risk Of Bias
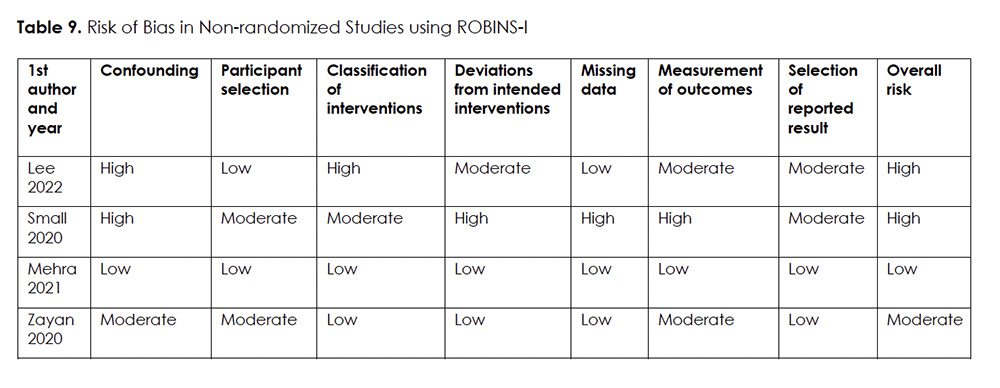
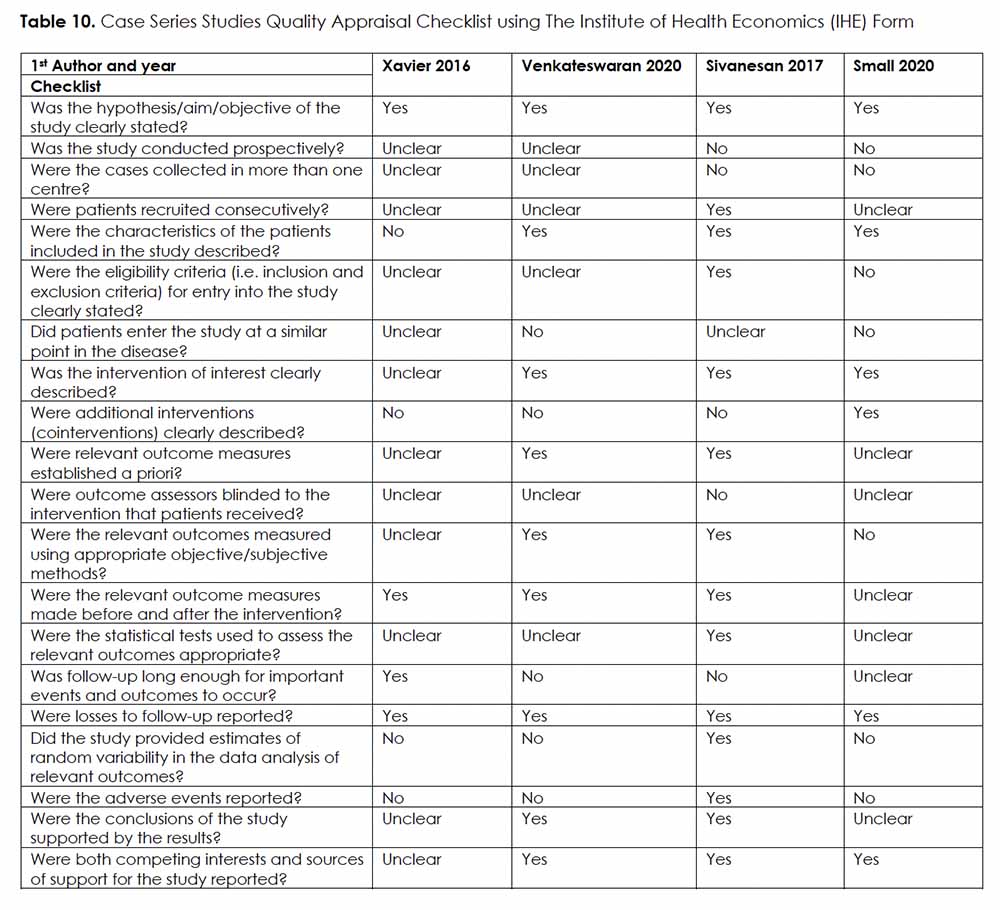
Two reviewers (S.A.A. and T.S.) independently evaluated risk of bias for non-randomized trials and case series using ROBINS-I 26 and IHE’s quality appraisal checklist for assessing case series studies 27, respectively. Any disagreement was resolved through discussion with the senior author (Y.H.).
Data Synthesis
We narratively synthetized the characteristics of all studies that met inclusion criteria. Study characteristics and treatment details were summarized. For continuous data, means (or medians) and standard deviations (or interquartile ranges or ranges) were extracted.
Results
Search Results
A total of 3925 unique articles were retrieved from the search, of which 3879 were excluded at the screening stage. Full texts of the remaining 46 articles were assessed with 12 studies meeting eligibility criteria (Figure 1). Four retrospective cohort studies 28-31, three case series 32-34, and five case reports 35-39 were included.
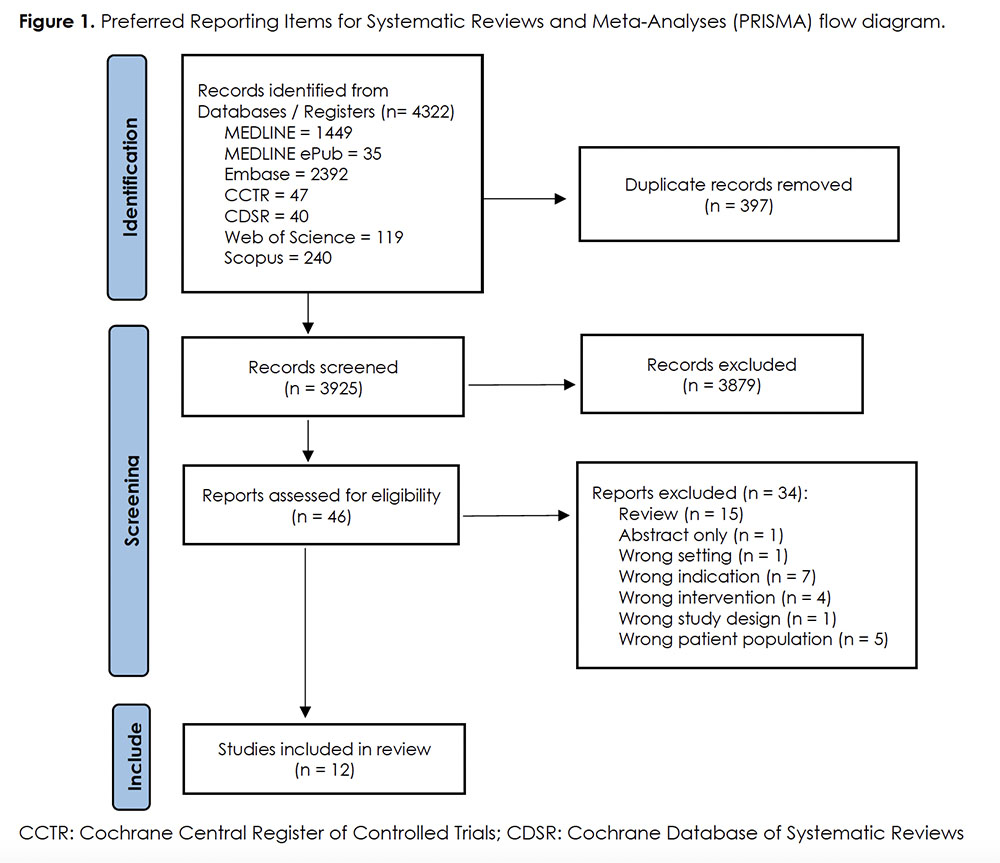
Risk Of Bias
The risk of bias assessment of the included non-randomized trials showed one study of low risk of bias 30, one study of moderate risk of bias 31, and two studies deemed to have a serious risk for bias 28, 29 (Table 9). The quality of case series was deemed low in two studies 29, 32, moderate in one study 33, and high in one study 34 (Table 10).
Interventions For NOP
From these 12 studies, eight interventions were identified for the treatment of NOP and listed below. We provide a concise rationale and indication for each intervention, summarize the data on treatment specifics and outcomes, and suggest potential areas for further research in this review.
Intervention 1: Stellate Ganglion Block (SGB) for NOP
Rationale:
The cervical sympathetic nervous system is responsible for the innervation of various structures in the body including blood vessels, sweat glands, eyes, face, head, neck, heart, and upper extremities. Several studies have shown that SGB may offer potential benefits for both painful and non-painful medical conditions. 40-42
Details Of The Studies And Outcomes:
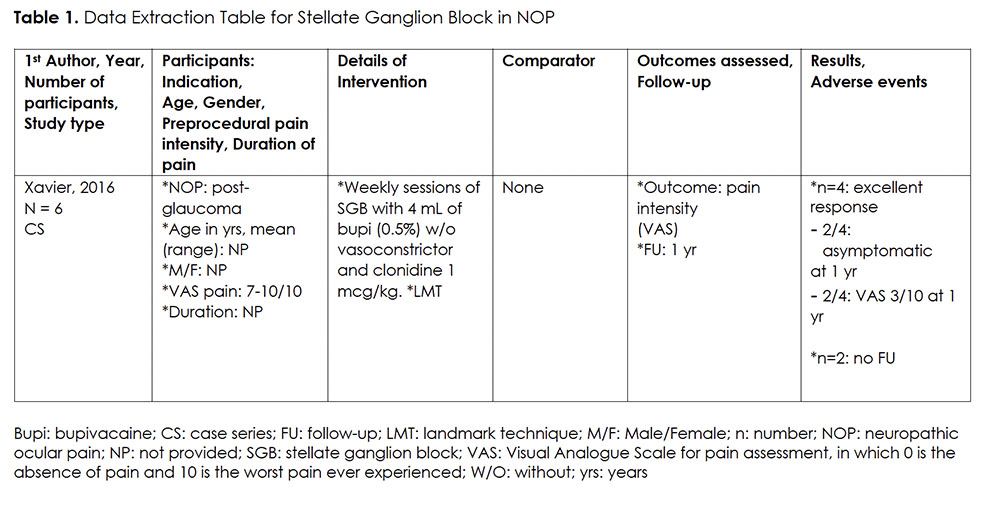
We only found one case series on the use of SGB for NOP (Table 1). 32 This small case series (n=6) investigated the effect of a course of six weekly sessions of SGB using landmark technique (LMT), injecting 4 ml of 0.5% bupivacaine and clonidine 1 mcg/kg, for participants suffering from NOP, caused by glaucoma. Participants’ preprocedural VAS (Visual Analogue Scale for Pain Assessment in which 0 is the absence of pain and 10 is the worst pain ever experienced) was high, averaging between 7-10/10. Four out of six participants reported a significant improvement. Two participants had complete pain relief up to one year after the procedure. Two other participants reported to only have mild pain, rated at 3/10, up to 12 months post-procedure. The latter group continued to take gabapentin simultaneously. Two of the six participants could not be evaluated due to loss of follow-up. There was no report on adverse events (AE).
Intervention 2: Peripheral Branches of Trigeminal Nerve Block (TNS) in NOP
Rationale:
Targeting the peripheral branches of the trigeminal nerve, including supraorbital, supratrochlear, infraorbital, and infratrochlear nerve, has been found to be an effective treatment for various conditions, such as migraine headaches, supratrochlear neuralgia, infratrochlear neuralgia, infraorbital neuralgia, and lacrimal neuralgia. 43-45 These periocular nerve blocks (PNB) have been suggested for the treatment of NOP based on the hypothesis that suggests that pain signals may arise due to the abnormal regeneration of damaged corneal nerve endings. This abnormal regeneration could lead to abnormal connections with adjacent nerve endings, resulting in spontaneous activity. The tissues surrounding the cornea, such as the palpebral conjunctiva, skin, or fornix, receive innervation from the supraorbital, supratrochlear, infratrochlear, and infraorbital nerves. 17 Therefore, blocking the periorbital nerves next to the injured corneal nerves could reduce ectopic activity and decrease pain signaling to the spinal trigeminal nucleus, leading to a reduction in eye pain perception. 29
Details Of The Studies And Outcomes:
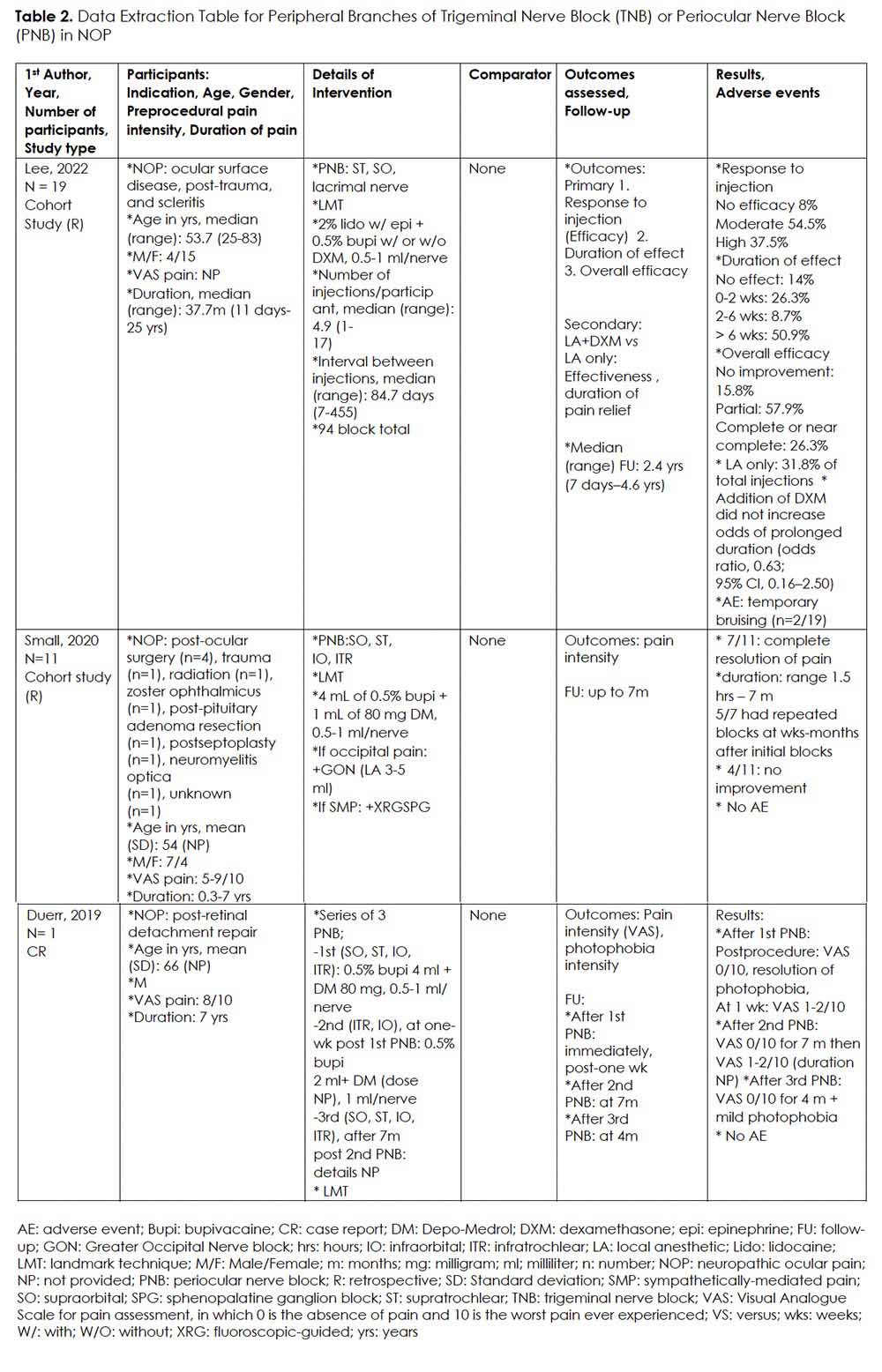
We found two retrospective cohort studies, 28, 29 and one case report 35 on the use of PNB for NOP (Table 2). The studies have included participants ranging from 37 to 69 years of age with moderate to severe intensity of NOP from different causes. Injectates consisted of local anesthetics alone or in combination with steroids. All procedures were done using LMT. In one study conducted by Lee et al., nineteen participants were given a total of 94 peripheral trigeminal nerve blocks. 28 The number of injections varied among the participants, with a median of 4.9 (range 1-17) injections per patient and a median of 84.7 days (range 7-455 days) between each injection. At a median follow-up period of 2.4 years (range 7 days – 4.6 years), the majority of participants (84.2%) reported that the injections continued to provide partial or complete pain improvement. Over half of those assessed reported effects lasting more than six weeks. Injections containing dexamethasone did not increase the odds of prolonged duration (relative risk, 0.88; 95% CI, 0.81-0.97).
In a complex study by Small et al., patients with severe NOP were treated with multi-modal analgesia including gabapentin. They found that adding gabapentin to multi-modal treatment regimen provided significant pain relief. 29 Eleven individuals who did not benefit from gabapentin, underwent PNB. Greater occipital nerve block or sphenopalatine ganglion block were added in case of occipital pain or sympathetically-mediated pain, respectively. Seven out of eleven individuals experienced complete resolution of pain lasting from 1.5 hours to 7 months. Repeated blocks were considered at weeks to months after initial blocks, if the pain recurred. No AE were reported.
Lastly, one participant reported by Duerr and colleagues in 2019 stated that they experienced significant pain relief and improvement of photophobia that lasted for several months (range 4-7) after each procedure. 35
Intervention 3: Inferotemporal Retrobulbar Injection in NOP
Rationale:
The retrobulbar block (RBB) was once the gold standard for akinesia and anesthesia in intraocular surgery, but its use has decreased due to newer techniques with similar efficacy and fewer complications. Although rare, complications such as retrobulbar hemorrhage, optic nerve damage, and central spread of local anesthetic and brainstem anesthesia can have severe consequences. 46
Details Of The Studies And Outcomes:
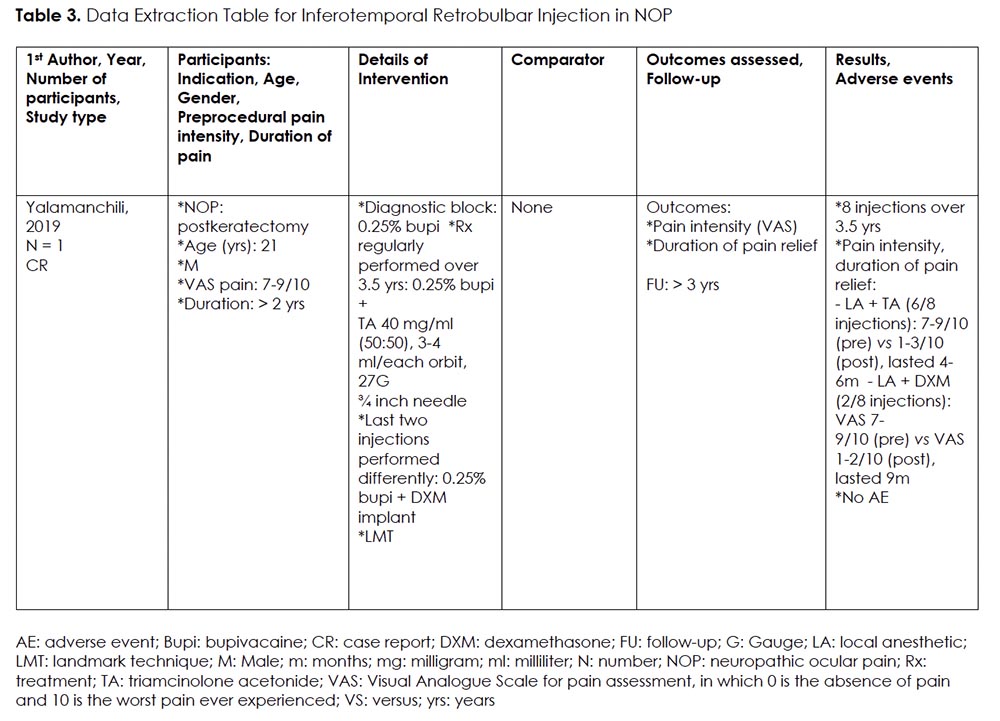
We found one case report of a young patient with NOP receiving landmark-guided (LMG) RBB. 36 (Table 3) Following a positive diagnostic block, he received 8 therapeutic injections over the course of 3.5 years, each providing him with significant pain reduction (VAS baseline 7-9/10 versus VAS post 1-3/10), lasting 4-9 months. The study reported longer duration of pain relief with dexamethasone as compared to triamcinolone acetonide (9 months versus 4-6 months). No complications were noted.
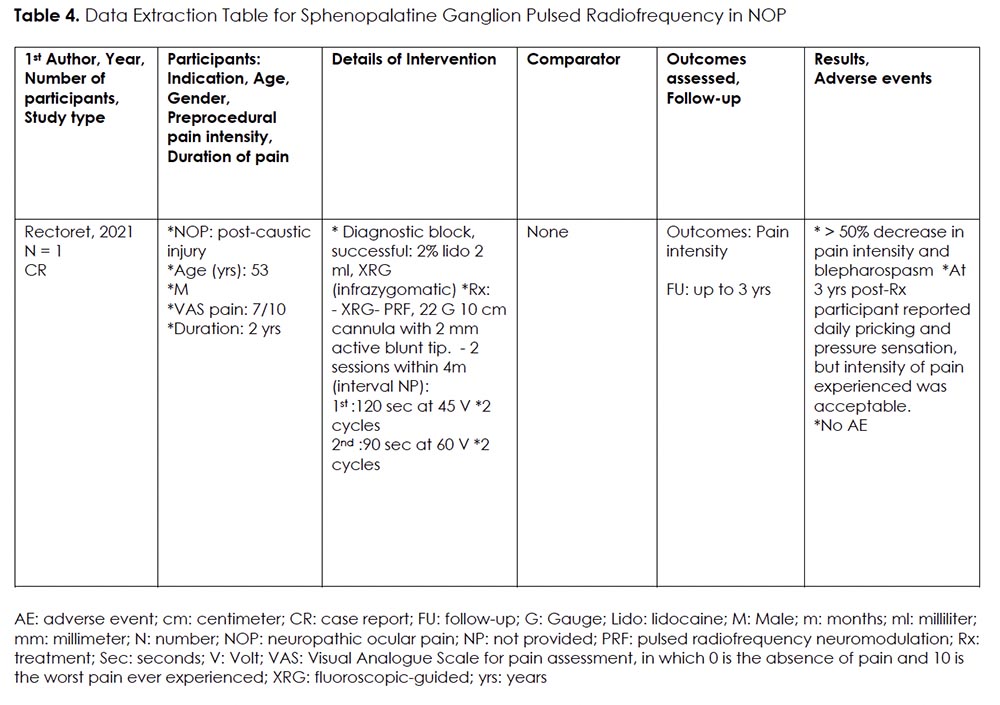
Intervention 4: Sphenopalatine Ganglion (SPG) Pulsed Radiofrequency Neuromodulation (PRFN) for NOP
Rationale:
The trigeminal-autonomic reflex is the most relevant signaling pathway in relation to SPG-mediated pain. Activation of this pathway leads to the release of vasoactive peptides that cause the extravasation of plasma proteins and neurogenic inflammation. 47, 48 Targeting the SPG with peri-target injection, radiofrequency ablation, and neurostimulation, have been studied and show promise in treating headache disorders, facial pain syndromes, and other facial neuralgias. 22, 49
Details Of The Studies And Outcomes:
We only found one case report on the use of PRFN of SPG for NOP. 37 (Table 4) The procedure was performed under fluoroscopic guidance on a 53-year-old male, who experienced refractory NOP with blepharospasm following caustic injury. Two sessions of PRFN of SPG were completed within 4 months’ time. The first session was performed for 120 seconds at 45 V for two cycles, and the second session performed for 90 seconds at 60 V for two cycles. After completion of both sessions, the participant reported a significant improvement in pain and blepharospasm symptoms, with still ongoing partial benefit at 3 years post-procedure.
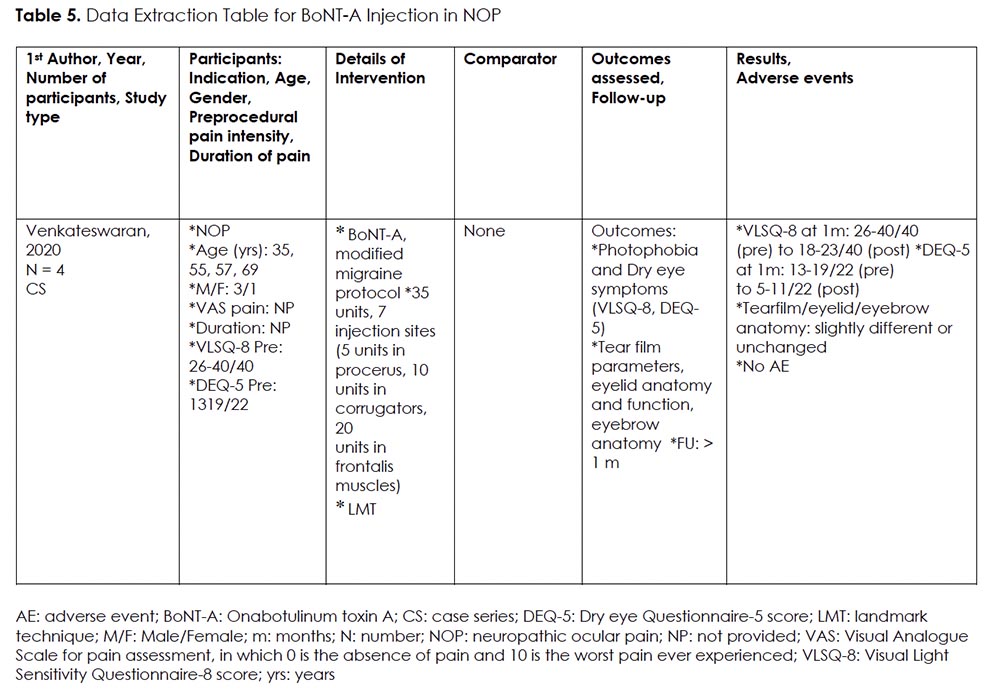
Intervention 5: BoNT-A Injection for NOP
Rationale:
Onabotulinum toxin A (BoNT-A) is a type of neurotoxin that is derived from Clostridium botulinum. It has been used as a therapeutic agent for a wide range of disorders such as cervical dystonia, chronic migraine, hyperhidrosis, urinary incontinence, strabismus, and blepharospasm. 50 Moreover, BoNT-A has been found to inhibit the release of local nociceptive neuropeptides such as substance P, calcitonin gene-related peptide (CGRP), and glutamate. 51 It also reduces the expression of transient receptor potential vanilloid 1 (TRPV1), thereby dampening neurogenic inflammation and peripheral sensitization. 52 Given these effects, BoNT-A has increasingly been used to treat a variety of neuropathic facial pain disorders, including post-herpetic neuralgia, trigeminal neuralgia, and occipital neuralgia. 53 One published case series 54, demonstrating that patients receiving BoNT-A injections for chronic migraine also experienced significant improvement in photophobia and DE, led to the hypothesis that individuals with NOP may experience similar symptomatic improvement with periocular BoNT-A injection.
Details Of The Studies And Outcomes:
We found one case series on the use of BoNT-A for refractory NOP (Table 5). 33 Patients received one session of periocular BoNT-A injection, using modified migraine protocol, targeting procerus, corrugators, and frontalis muscles. The rationale was to target the muscles closest to trigeminal afferents on the corneal surface. The severity and frequency of photophobia and eye discomfort were assessed, using the Visual Light Sensitivity Questionnaire-8 (VLSQ-8) 55 and Dry Eye Questionnaire-5 (DEQ-5) 56. Both parameters were demonstrated to be significantly decreased (VLSQ-8 scores pre: 26-40/40, post: 18-23/40; DEQ-5 scores pre:13-19/22, post: 5-11/22). Tear film parameters, eyelid, and eyebrow anatomy were also evaluated but deemed unchanged.
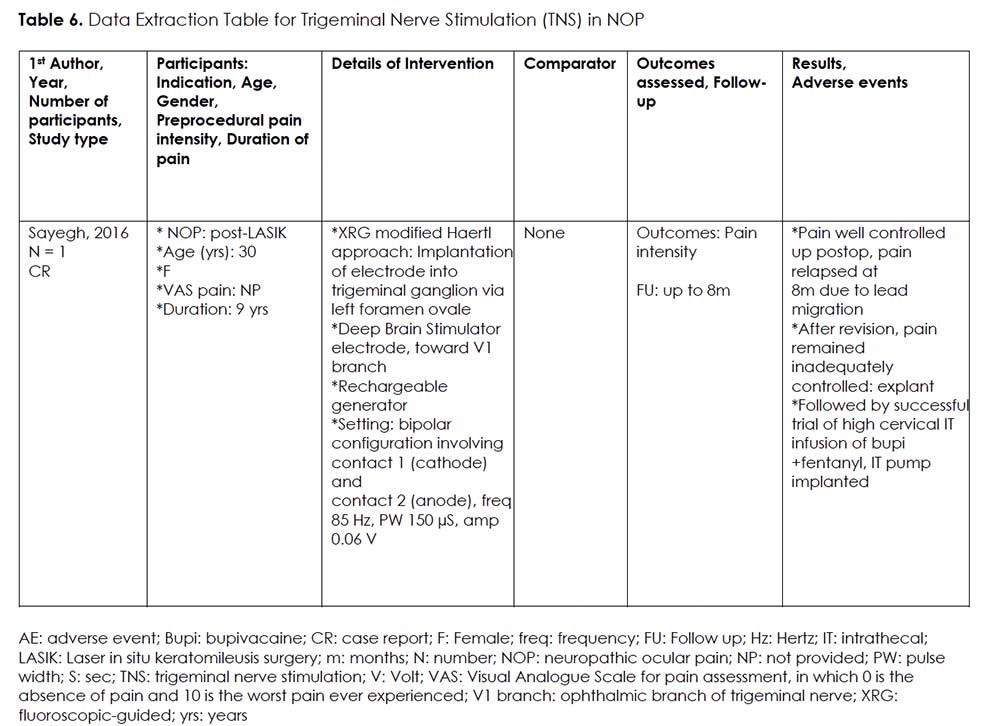
Intervention 6: Trigeminal Nerve Stimulation (TNS) for NOP
Rationale:
According to the neurophysiological gate-control theory proposed by Melzack and Wall, the stimulation of large-diameter afferent fibers inhibits the transmission of noxious stimuli by small-diameter fibers. 57 Subsequently, invasive stimulation of the trigeminal nerves through the Gasserian ganglion has been investigated for the treatment of chronic atypical trigeminal neuralgia. 58 Similarly, NOP has been suggested as a possible indication for TNS.
Details Of The Studies And Outcomes:
We found one case report describing a 30-year-old woman experiencing severe DE-like symptoms and NOP post laser in situ keratomileusis surgery (LASIK). 38 (Table 6) The participant underwent fluoroscopic-guided implantation of an electrode close to the first trigeminal branch (V1). This procedure provided significant pain control until lead migration at 8 months post-implant. Further attempts to revise the implant failed to provide adequate pain control, and the device was explanted. The same patient then received an intrathecal drug delivery system (IDDS) with fentanyl and bupivacaine at C1-C2 level, providing adequate symptom control for over a year. It is worth noting that this study was related to another publication by Hayek et al., 39 but the latter focused more on intrathecal drug delivery system, discussed in Intervention 7 section.
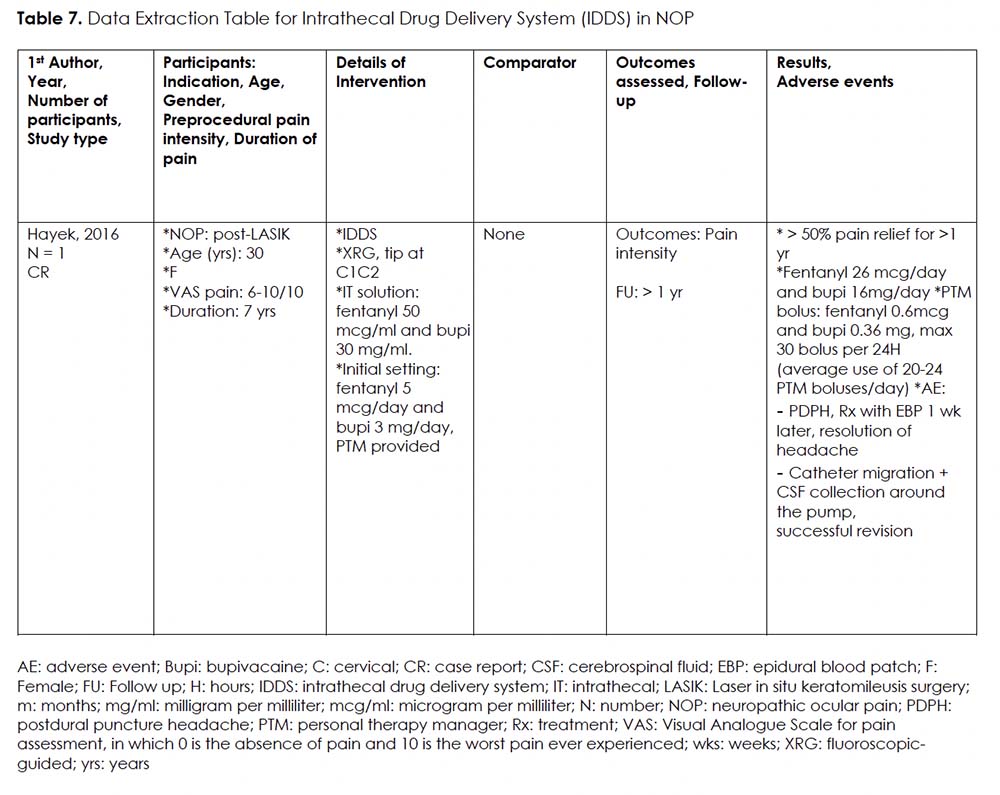
Intervention 7: Intrathecal Drug Delivery System (IDDS) for NOP
Rationale:
Lundborg et al. conducted a study from 1990 to 2005 on use of continuous high intrathecal bupivacaine administration to treat 40 patients with refractory pain in the head, neck, mouth, and shoulder regions; cancer-related (n=25) and non-cancer (n=15). 59 The study was based on clinical experiences and promising results from case reports. 60-62 They concluded that cervical high spinal analgesia is a safe and effective treatment for refractory pain in areas innervated by cranial and upper cervical nerves. The results showed significant pain relief and reduced opioid requirement with few side effects.
Details Of The Studies And Outcomes:
We only found one case report on the use of IDDS for NOP. 39 (Table 7) The tip of the intrathecal catheter was located at C1-C2 level. Patient satisfaction was high with over 50% pain relief for more than a year. The continuous infusion was started at fentanyl 5 mcg/day and bupivacaine 3 mg/day, and titrated up to fentanyl 26 mcg/day and bupivacaine 16 mg/day. Average frequency of bolus use was 20-24 times per day. Documented complications included post-dural puncture headache and catheter migration. It is crucial to note that appropriate catheter positioning at the C1-C2 level was vital for relieving NOP in this case, as evidenced by the loss of analgesia when the catheter migrated 2 cm caudad.
Intervention 8: Transcutaneous Electrical Nerve Stimulation (TENS) for NOP
Rationale:
Studies have shown that TENS is effective in treating various pain conditions, including fibromyalgia, painful diabetic neuropathy, migraines, facial pain. 63-70 There are two major theories explaining TENS’ analgesic mechanism: Gate Control Theory and descending inhibitory pathway modulation. 57, 71 High frequency TENS (>60 Hz) has been shown to activate supraspinal delta-opioid and cholinergic receptors, modifying the release of gamma-aminobutyric acid (GABA) and enkephalins, which facilitate inhibition of interneurons within the trigeminal-thalamic tract in the context of ocular pain. 72-74
Details Of The Studies And Outcomes:
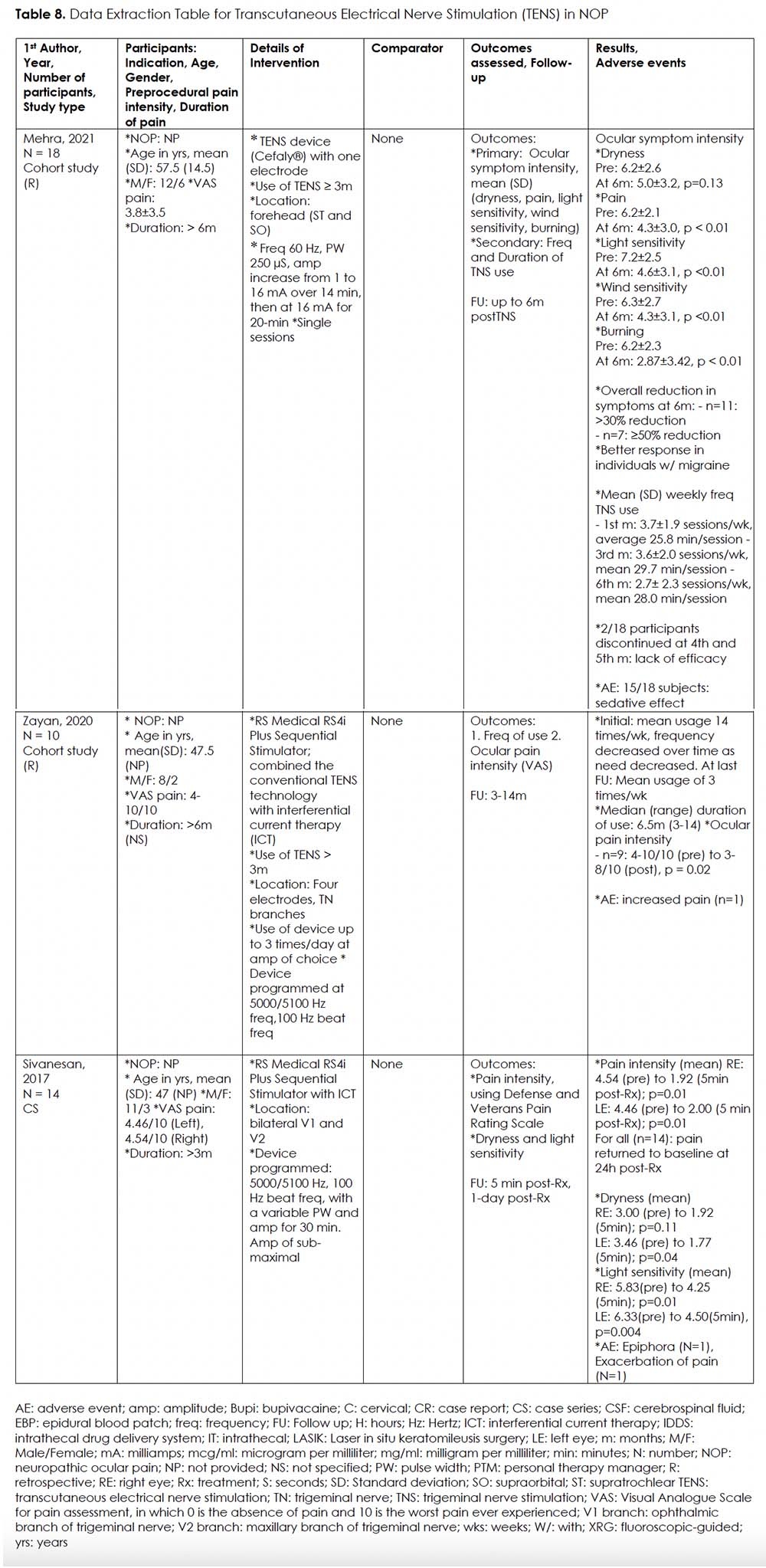
We came across two retrospective studies 30, 31 and a case series 34 on use of TENS for NOP. (Table 8) Two studies utilized the RS Medical RS-4i Plus Sequential Stimulator, a device that combines traditional TENS technology with interferential current therapy (ICT) to potentially reduce adverse dysesthesias that are commonly associated with traditional TENS stimulation. 31, 34 The other study used the Compact Trigeminal NeuroStimulator (TNS) device (Cefaly®) with a single electrode. 30 Electrodes were attached to the forehead and temples for RS-4i, and center of the forehead for TNS device, covering the ophthalmic (V1) and maxillary (V2) branches.
In one retrospective cohort study (n=18) investigating the use of Cefaly® TENS device, patients were instructed to use the device daily for at least 20 minutes, continued for at least 3 months. 30 Ocular symptom intensity including dryness, pain, light sensitivity, wind sensitivity, and burning were evaluated monthly for 6 months. Except for dryness, all other ocular symptoms were significantly decreased up to 6 months. Mean weekly frequency use of TNS decreased over time, though therapeutic effect remained. Interestingly, the majority of participants (15/18) reported feeling sedated when TNS use. A second retrospective cohort study (n=10) investigated the use of RS-4i. 31 Follow-up ranged from 3 to 14 months. Nine out of ten patients reported significant pain relief at last follow-up. Interestingly, both studies reported that mean weekly frequency use of TENS decreased over time, indicative of a lower need for treatment.
Finally, we found one case series (n=13) reporting significant but short-lasing decrease in ocular pain intensity following a single episode of 30-minute session with the RS-4i device. 34 Participants’ ocular symptoms returned to baseline within 24 hours post-treatment. Adverse effects reported were epiphora (n = 1) and exacerbation of pain (n = 1).
Discussion
NOP is a highly complex and refractory pain condition. To our knowledge, this is the first scoping review providing a detailed summary of the rationale and current evidence for the use of various pain interventions to manage NOP. We only found low-quality evidence to support the use of eight interventions, with moderate risks of bias.
The study of NOP is becoming increasingly important as we seek to understand its complex underlying mechanism. This may include autonomic, peripheral and central sensitization components, or a combination of all three. Despite an extensive list of non-pharmacological options (such as lifestyle modification and cognitive behavioral therapy), pharmacological options (such as topical therapy, gabapentinoid, serotonin and norepinephrine reuptake inhibitor, tricyclic antidepressant, anti-convulsant, low-dose naltrexone) and the included interventional options, finding the optimal treatment for these refractory patients remains challenging and is probably multi-faceted and multidisciplinary. Therefore, encouraging collaboration amongst different specialties and adopting a multimodality approach would be the most effective strategy at this time. In that context, we hope our scoping review will bring some new insights.
This review has several limitations. All reported evidence stems from observational data, that is flawed by high risk of confounding and bias. This becomes even more apparent due to significant heterogeneity, lack of long-term follow-up and a low number of studies for each intervention. This prohibits us from making any recommendations at this time.
Nevertheless, this review has revealed some promising data and should be seen as a boost and call for development of high-quality evidence with monitoring of long-term outcomes and adverse effects to clearly delineate the role of these treatments in this refractory patient population that is desperately in need of better pain control.
Conclusion
NOP is a complex and refractory pain condition. The current evidence for interventional treatment for NOP is limited and of low quality, offering insufficient support to provide recommendations. Given the debilitating character of this disease, there is an urgent need for high-quality studies, including monitoring of long-term outcomes and adverse effects, to clearly establish the efficacy of included pain interventions for NOP.
Acknowledgment
The authors would like to express gratitude to Ms. Marina Englesakis, the information specialist at the University Health Network in Toronto, for her dedicated assistance with the literature search.
Tables
References
- Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf. Jan 2017;15(1):15-47. PubMed PMID: 27771327; PubMed Central PMCID: PMCPMC5512932. doi:10.1016/j.jtos.2016.09.004
- Sanchez V, Cohen NK, Felix E, Galor A. Factors affecting the prevalence, severity, and characteristics of ocular surface pain. Expert Rev Ophthalmol. 2023;18(1):19-32. PubMed PMID: 37009062; PubMed Central PMCID: PMCPMC10062703. doi:10.1080/17469899.2023.2157813
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. Jul 2017;15(3):334-365. PubMed PMID: 28736337. doi:10.1016/j.jtos.2017.05.003
- Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. Jun 2009;127(6):763-8. PubMed PMID: 19506195; PubMed Central PMCID: PMCPMC2836718. doi:10.1001/archophthalmol.2009.103
- Galor A, Zlotcavitch L, Walter SD, et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. Br J Ophthalmol. May 2015;99(5):665-8. PubMed PMID: 25336572. doi:10.1136/bjophthalmol-2014-306057
- Morthen MK, Magno MS, Utheim TP, Snieder H, Hammond CJ, Vehof J. The physical and mental burden of dry eye disease: A large population-based study investigating the relationship with health-related quality of life and its determinants. Ocul Surf. Jul 2021;21:107-117. PubMed PMID: 34044135. doi:10.1016/j.jtos.2021.05.006
- Sayegh RR, Yu Y, Farrar JT, et al. Ocular Discomfort and Quality of Life Among Patients in the Dry Eye Assessment and Management Study. Cornea. Jul 1 2021;40(7):869-876. PubMed PMID: 33290317; PubMed Central PMCID: PMCPMC8175479. doi:10.1097/ICO.0000000000002580
- Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain–Gaps and Current Therapeutic Approaches. Semin Ophthalmol. 2016;31(1-2):59-70. PubMed PMID: 26959131; PubMed Central PMCID: PMCPMC5607443. doi:10.3109/08820538.2015.1114853
- Patel S, Mittal R, Sarantopoulos KD, Galor A. Neuropathic ocular surface pain: Emerging drug targets and therapeutic implications. Expert Opin Ther Targets. Aug 2022;26(8):681-695. PubMed PMID: 36069761; PubMed Central PMCID: PMCPMC9613591. doi:10.1080/14728222.2022.2122438
- Rosenthal P, Borsook D, Moulton EA. Oculofacial Pain: Corneal Nerve Damage Leading to Pain Beyond the Eye. Invest Ophthalmol Vis Sci. Oct 1 2016;57(13):5285-5287. PubMed PMID: 27723896; PubMed Central PMCID: PMCPMC5063054. doi:10.1167/iovs.16-20557
- Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. Jan 2016;100(1):128-34. PubMed PMID: 25943558; PubMed Central PMCID: PMCPMC4717373. doi:10.1136/bjophthalmol-2014-306280
- Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. Feb 2008;115(2 Suppl):S3-12. PubMed PMID: 18243930. doi:10.1016/j.ophtha.2007.10.009
- Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf. Jan 2009;7(1):28-40. PubMed PMID: 19214350. doi:10.1016/s1542-0124(12)70290-2
- Theophanous C, Jacobs DS, Hamrah P. Corneal Neuralgia after LASIK. Optom Vis Sci. Sep 2015;92(9):e233-40. PubMed PMID: 26154691. doi:10.1097/OPX.0000000000000652
- Nettune GR, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocul Surf. Jul 2010;8(3):135-45. PubMed PMID: 20712970; PubMed Central PMCID: PMCPMC3579556. doi:10.1016/s1542-0124(12)70224-0
- Dieckmann G, Goyal S, Hamrah P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology. Nov 2017;124(11S):S34-S47. PubMed PMID: 29055360; PubMed Central PMCID: PMCPMC5743225. doi:10.1016/j.ophtha.2017.08.004
- Ebrahimiadib N, Yousefshahi F, Abdi P, Ghahari M, Modjtahedi BS. Ocular Neuropathic Pain: An Overview Focusing on Ocular Surface Pains. Clin Ophthalmol. 2020;14:2843-2854. PubMed PMID: 33061269; PubMed Central PMCID: PMCPMC7524198. doi:10.2147/OPTH.S262060
- Kalangara JP, Galor A, Levitt RC, et al. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens. May 2017;43(3):192-198. PubMed PMID: 26925537; PubMed Central PMCID: PMCPMC5003761. doi:10.1097/ICL.0000000000000249
- Wie C, Gupta R, Maloney J, Pew S, Freeman J, Strand N. Interventional Modalities to Treat Complex Regional Pain Syndrome. Curr Pain Headache Rep. Feb 3 2021;25(2):10. PubMed PMID: 33537907. doi:10.1007/s11916-020-00904-5
- Duong S, Bravo D, Todd KJ, Finlayson RJ, Tran Q. Treatment of complex regional pain syndrome: an updated systematic review and narrative synthesis. Can J Anaesth. Jun 2018;65(6):658-684. Traitement du syndrome douloureux regional complexe : etude systematique actualisee et synthese narrative. PubMed PMID: 29492826. doi:10.1007/s12630-018-1091-5
- Lin CS, Lin YC, Lao HC, Chen CC. Interventional Treatments for Postherpetic Neuralgia: A Systematic Review. Pain Physician. May 2019;22(3):209-228. PubMed PMID: 31151330.
- Ho KWD, Przkora R, Kumar S. Sphenopalatine ganglion: block, radiofrequency ablation and neurostimulation – a systematic review. J Headache Pain. Dec 28 2017;18(1):118. PubMed PMID: 29285576; PubMed Central PMCID: PMCPMC5745368. doi:10.1186/s10194-017-0826-y
- Hary V, Schitter S, Martinez V. Efficacy and safety of botulinum A toxin for the treatment of chronic peripheral neuropathic pain: A systematic review of randomized controlled trials and meta-analysis. Eur J Pain. May 2022;26(5):980-990. PubMed PMID: 35293078. doi:10.1002/ejp.1941
- Arksey HOM, L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32. doi:https://doi.org/10.1080/1364557032000119616
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. Sep 20 2010;5:69. PubMed PMID: 20854677; PubMed Central PMCID: PMCPMC2954944. doi:10.1186/1748-5908-5-69
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. Oct 12 2016;355:i4919. PubMed PMID: 27733354; PubMed Central PMCID: PMCPMC5062054. doi:10.1136/bmj.i4919
- Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol. Jan 2016;69:199-207 e2. PubMed PMID: 26307459. doi:10.1016/j.jclinepi.2015.07.010
- Lee G, Pham CM, Kardon RH, Shriver EM. Peripheral Trigeminal Nerve Blocks for Chronic Orbital Pain: Clinical Features and Outcomes. Ophthalmic Plast Reconstr Surg. Jul-Aug 01 2022;38(4):369-376. PubMed PMID: 35030151. doi:10.1097/IOP.0000000000002120
- Small LR, Galor A, Felix ER, Horn DB, Levitt RC, Sarantopoulos CD. Oral Gabapentinoids and Nerve Blocks for the Treatment of Chronic Ocular Pain. Eye Contact Lens. May 2020;46(3):174-181. PubMed PMID: 31206369. doi:10.1097/ICL.0000000000000630
- Mehra D, Mangwani-Mordani S, Acuna K, J CH, E RF, Galor A. Long-Term Trigeminal Nerve Stimulation as a Treatment for Ocular Pain. Neuromodulation. Aug 2021;24(6):1107-1114. PubMed PMID: 33945660. doi:10.1111/ner.13402
- Zayan K, Aggarwal S, Felix E, Levitt R, Sarantopoulos K, Galor A. Transcutaneous Electrical Nerve Stimulation for the Long-Term Treatment of Ocular Pain. Neuromodulation. Aug 2020;23(6):871-877. PubMed PMID: 32196838; PubMed Central PMCID: PMCPMC7483841. doi:10.1111/ner.13146
- Xavier TV, de Oliveira TR, Mendes TC. Treatment of patients with painful blind eye using stellate ganglion block. Braz J Anesthesiol. Jan-Feb 2016;66(1):75-7. PubMed PMID: 26768934. doi:10.1016/j.bjane.2012.12.009
- Venkateswaran N, Hwang J, Rong AJ, et al. Periorbital botulinum toxin A improves photophobia and sensations of dryness in patients without migraine: Case series of four patients. Am J Ophthalmol Case Rep. Sep 2020;19:100809. PubMed PMID: 32671286; PubMed Central PMCID: PMCPMC7350146. doi:10.1016/j.ajoc.2020.100809
- Sivanesan E, Levitt RC, Sarantopoulos CD, Patin D, Galor A. Noninvasive Electrical Stimulation for the Treatment of Chronic Ocular Pain and Photophobia. Neuromodulation. Dec 2018;21(8):727-734. PubMed PMID: 29283468; PubMed Central PMCID: PMCPMC6023783. doi:10.1111/ner.12742
- Duerr ER, Chang A, Venkateswaran N, et al. Resolution of pain with periocular injections in a patient with a 7-year history of chronic ocular pain. Am J Ophthalmol Case Rep. Jun 2019;14:35-38. PubMed PMID: 30815622; PubMed Central PMCID: PMCPMC6378870. doi:10.1016/j.ajoc.2019.02.001
- Yalamanchili SP, Hertle RW. Treatment of Ocular Neuralgia After Refractive Surgery With Bilateral Orbital Steroid and Anesthetic Injections. J Refract Surg. Aug 1 2019;35(8):534-537. PubMed PMID: 31393992. doi:10.3928/1081597X-20190722-01
- Rectoret SS S, VF, Hurtado, GR, Tabasco, MM. Radiofrecuencia del ganglio esfenopalatino en caso de dolor ocular refractario a tratamiento conservador. Dolor Investigación Clínica & Terapéutica. 2021;36(2):104-8.
- Sayegh RR, Sweet JA, Miller JP, Hayek SM. Electrical Stimulation of the Trigeminal Ganglion and Intrathecal Drug Delivery Systems for the Management of Corneal Neuropathic Pain. Cornea. Apr 2016;35(4):576-7. PubMed PMID: 26807903. doi:10.1097/ICO.0000000000000751
- Hayek SM, Sweet JA, Miller JP, Sayegh RR. Successful Management of Corneal Neuropathic Pain with Intrathecal Targeted Drug Delivery. Pain Med. Jul 2016;17(7):1302-7. PubMed PMID: 26814286. doi:10.1093/pm/pnv058
- Baig S, Moon JY, Shankar H. Review of Sympathetic Blocks: Anatomy, Sonoanatomy, Evidence, and Techniques. Reg Anesth Pain Med. May/Jun 2017;42(3):377-391. PubMed PMID: 28272291. doi:10.1097/AAP.0000000000000591
- Tumber P J, D. Cervical Sympathetic Chain and Superior Cervical Ganglion Block. Regional Nerve Blocks in Anesthesia and Pain Therapy. Springer; 2022.
- Feigin G, Velasco Figueroa S, Englesakis MF, D’Souza R, Hoydonckx Y, Bhatia A. Stellate ganglion block for non-pain indications: a scoping review. Pain Med. Jul 5 2023;24(7):775-781. PubMed PMID: 36727500. doi:10.1093/pm/pnad011
- Ilhan Alp S, Alp R. Supraorbital and infraorbital nerve blockade in migraine patients: results of 6-month clinical follow-up. Eur Rev Med Pharmacol Sci. Jul 2013;17(13):1778-81. PubMed PMID: 23852904.
- Pareja JA, Lopez-Ruiz P, Mayo D, et al. Supratrochlear Neuralgia: A Prospective Case Series of 15 Patients. Headache. Oct 2017;57(9):1433-1442. PubMed PMID: 28833061. doi:10.1111/head.13158
- Villar-Quiles RN, Garcia-Moreno H, Mayo D, et al. Infratrochlear neuralgia: A prospective series of seven patients treated with infratrochlear nerve blocks. Cephalalgia. Mar 2018;38(3):585-591. PubMed PMID: 28114806. doi:10.1177/0333102417690493
- Polania Gutierrez JJ, Riveros Perez E. Retrobulbar Block. StatPearls. 2023.
- Robbins MS, Robertson CE, Kaplan E, et al. The Sphenopalatine Ganglion: Anatomy, Pathophysiology, and Therapeutic Targeting in Headache. Headache. Feb 2016;56(2):240-58. PubMed PMID: 26615983. doi:10.1111/head.12729
- Mojica J, Mo B, Ng A. Sphenopalatine Ganglion Block in the Management of Chronic Headaches. Curr Pain Headache Rep. Jun 2017;21(6):27. PubMed PMID: 28432602. doi:10.1007/s11916-017-0626-8
- Tolba R, Weiss AL, Denis DJ. Sphenopalatine Ganglion Block and Radiofrequency Ablation: Technical Notes and Efficacy. Ochsner J. Spring 2019;19(1):32-37. PubMed PMID: 30983899; PubMed Central PMCID: PMCPMC6447206. doi:10.31486/toj.18.0163
- Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. Sep 1981;33(3):155-88. PubMed PMID: 6119708.
- Oh HM, Chung ME. Botulinum Toxin for Neuropathic Pain: A Review of the Literature. Toxins (Basel). Aug 14 2015;7(8):3127-54. PubMed PMID: 26287242; PubMed Central PMCID: PMCPMC4549742. doi:10.3390/toxins7083127
- Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. Oct 2005;26(5):785-93. PubMed PMID: 16002144. doi:10.1016/j.neuro.2005.01.017
- Jeynes LC, Gauci CA. Evidence for the use of botulinum toxin in the chronic pain setting–a review of the literature. Pain Pract. Jul-Aug 2008;8(4):269-76. PubMed PMID: 18503628. doi:10.1111/j.1533-2500.2008.00202.x
- Diel RJ, Kroeger ZA, Levitt RC, et al. Botulinum Toxin A for the Treatment of Photophobia and Dry Eye. Ophthalmology. Jan 2018;125(1):139-140. PubMed PMID: 29110944; PubMed Central PMCID: PMCPMC5741464. doi:10.1016/j.ophtha.2017.09.031
- Verriotto JD, Gonzalez A, Aguilar MC, et al. New Methods for Quantification of Visual Photosensitivity Threshold and Symptoms. Transl Vis Sci Technol. Jul 2017;6(4):18. PubMed PMID: 28845363; PubMed Central PMCID: PMCPMC5566267. doi:10.1167/tvst.6.4.18
- Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. Apr 2010;33(2):55-60. PubMed PMID: 20093066. doi:10.1016/j.clae.2009.12.010
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. Nov 19 1965;150(3699):971-9. PubMed PMID: 5320816. doi:10.1126/science.150.3699.971
- Holsheimer J. Electrical stimulation of the trigeminal tract in chronic, intractable facial neuralgia. Arch Physiol Biochem. Oct 2001;109(4):304-8. PubMed PMID: 11935364. doi:10.1076/apab.109.4.304.4246
- Lundborg C, Dahm P, Nitescu P, Biber B. High intrathecal bupivacaine for severe pain in the head and neck. Acta Anaesthesiol Scand. Aug 2009;53(7):908-13. PubMed PMID: 19456301. doi:10.1111/j.1399-6576.2009.01989.x
- Baker L, Balls J, Regnard C, Pridie A. Cervical intrathecal analgesia for head and neck/upper limb cancer pain: six case reports. Palliat Med. Sep 2007;21(6):543-5. PubMed PMID: 17846095. doi:10.1177/0269216307081130
- Narvaez MJ, Bulnes JM, Elena JM, Rivas JM, Marquez BM. Programmable pump for the administration of morphine in the cisterna magna. A new approach. Neuromodulation. Jul 2002;5(3):145-9. PubMed PMID: 22150811. doi:10.1046/j.1525-1403.2002.02024.x
- Crul BJ, van Dongen RT, Snijdelaar DG, Rutten EH. Long-term continuous intrathecal administration of morphine and bupivacaine at the upper cervical level: access by a lateral C1-C2 approach. Anesth Analg. Sep 1994;79(3):594-7. PubMed PMID: 8067573. doi:10.1213/00000539-199409000-00036
- Gibson W, Wand BM, O’Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst Rev. Sep 14 2017;9(9):CD011976. PubMed PMID: 28905362; PubMed Central PMCID: PMCPMC6426434. doi:10.1002/14651858.CD011976.pub2
- Johnson MI, Claydon LS, Herbison GP, Jones G, Paley CA. Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults. Cochrane Database Syst Rev. Oct 9 2017;10(10):CD012172. PubMed PMID: 28990665; PubMed Central PMCID: PMCPMC6485914. doi:10.1002/14651858.CD012172.pub2
- Naderi Nabi B, Sedighinejad A, Haghighi M, et al. Comparison of Transcutaneous Electrical Nerve Stimulation and Pulsed Radiofrequency Sympathectomy for Treating Painful Diabetic Neuropathy. Anesth Pain Med. Oct 2015;5(5):e29280. PubMed PMID: 26587405; PubMed Central PMCID: PMCPMC4644305. doi:10.5812/aapm.29280
- Ozkul C, Kilinc M, Yildirim SA, Topcuoglu EY, Akyuz M. Effects of visual illusion and transcutaneous electrical nerve stimulation on neuropathic pain in patients with spinal cord injury: A randomised controlled cross-over trial. J Back Musculoskelet Rehabil. 2015;28(4):709-19. PubMed PMID: 25502348. doi:10.3233/BMR-140573
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. Aug 2010;9(8):807-19. PubMed PMID: 20650402. doi:10.1016/S1474-4422(10)70143-5
- Gupta R, Fisher K, Pyati S. Chronic Headache: a Review of Interventional Treatment Strategies in Headache Management. Curr Pain Headache Rep. Jul 29 2019;23(9):1-9. PubMed PMID: 31359257. doi:10.1007/s11916-019-0806-9
- Zayan K, Felix ER, Galor A. Transcutaneous Electrical Nerve Stimulation for Facial Pain. Prog Neurol Surg. 2020;35:35-44. PubMed PMID: 32694253. doi:10.1159/000509620
- Subramonian A, Argaez C. Non-invasive Nerve Stimulation Modalities for Migraine Pain: A Review of Clinical Effectiveness and Cost-effectiveness. 2020. CADTH Rapid Response Reports.
- Ainsworth L, Budelier K, Clinesmith M, et al. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. Jan 2006;120(1-2):182-187. PubMed PMID: 16360266. doi:10.1016/j.pain.2005.10.030
- Maeda Y, Lisi TL, Vance CG, Sluka KA. Release of GABA and activation of GABA(A) in the spinal cord mediates the effects of TENS in rats. Brain Res. Mar 9 2007;1136(1):43-50. PubMed PMID: 17234163; PubMed Central PMCID: PMCPMC2746639. doi:10.1016/j.brainres.2006.11.061
- Radhakrishnan R, Sluka KA. Spinal muscarinic receptors are activated during low or high frequency TENS-induced antihyperalgesia in rats. Neuropharmacology. Dec 2003;45(8):1111-9. PubMed PMID: 14614954; PubMed Central PMCID: PMCPMC2746650. doi:10.1016/s0028-3908(03)00280-6
- Radhakrishnan R, King EW, Dickman JK, et al. Spinal 5-HT(2) and 5-HT(3) receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain. Sep 2003;105(1-2):205-13. PubMed PMID: 14499437; PubMed Central PMCID: PMCPMC2746627. doi:10.1016/s0304-3959(03)00207-0
Declarations/Disclosures
Consent/Permission/Ethics Approval: Not indicated based on the type of manuscript
Funding/Conflicts of interest: In compliance with the ICMJE uniform disclosure form, author declares the following:
Payment/services info: Author has declared that no financial support was received from any organization for the submitted work.
Financial relationships: Author have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: Author have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Authorship statement: Conception and design: YH and ME; Research into, design of, and execution of database search strategies: ME; Management and curation of database results, write up of search methodology; ME; Data acquisition, analysis & interpretation: YH, S.A.A., T.S.; Drafting of the article: YH and S.A.A; Review and editing of manuscript: all.
REB: Not indicated based on the type of manuscript
