Author: Bert B. Vargas * MD 1, Katherine J. Selzler PhD 1, Lauren Madar BA 1, Jeanine Brosch BS 1, William R. Prucka PhD 1, Rahul Singhal MCA 2, Robert A. Nicholson PhD 1
Author Affiliation:
1 Eli Lilly and Company, Indianapolis, IN, USA
2 TCS, Indianapolis, IN, USA
Competing Interests: The author/s declare no competing interests.
Issue: 06.02
DOI: 10.30756/ahmj.2021.06.02
Received: Sept 27, 2021
Revised: Dec 14, 2021
Accepted: Dec 16, 2021
Published: Jan 5, 2022
Recommended Citation: Vargas BB, Selzler KJ, Madar L, Brosch J, Prucka WR, Singhal R, Nicholson RA. A Human Factors Study Exploring the Experience of Participants Using VEGA, a Smartphone Migraine Management Application. Ann Head Med. 2021;06:02. DOI: 10.30756/ahmj.2021.06.02
Background and objectives: There is increasing interest by patients and healthcare providers in using digital tools such as smartphone applications to log migraine days, potential triggers and medication use. However, there are opportunities to optimize the learnability and ease of use of these applications. In this human factors study, we evaluated the experience of individuals with migraine using the VEGA mobile application.
Material and methods: For 4 weeks, individuals diagnosed with migraine used the VEGA application, which generated a migraine report containing 4 weeks of data, where migraine days were reported based on functional impact, pain severity, and medication use. Participants also responded to a survey and rated the ease of use of the application using a 6-point Likert scale, with “very easy” being the highest ranking.
Results: A total of 22 participants completed this study, ranged between 22 to 57 years old, and 54% were males. Eighteen participants completed the migraine report and logged 160 total migraine days. Participants reported the functional impact of migraine as “mild,” “moderate,” or “severe” for respectively 2, 44, and 50 of the logged migraine days. The pain severity was scored as “mild” for 24 days, “moderate” for 65 days, and “severe” for 63 days. Moreover, 20 participants reported it was “easy” or “very easy” to learn how to use the application, and 19 participants found it “simple” or “extremely simple” to use.
Conclusions: In this human factors study, the VEGA application was easy to use, providing real-time patient-reported data.
Introduction
Migraine is a neurological disease, causing disability in over 1 billion people worldwide 1. Despite a wide choice of available treatments 2, migraine remains underrecognized and undertreated 3, 4. Incorrectly or incompletely reported headache features and characteristics may contribute 5, by reducing the provider’s ability to accurately diagnose migraine and/or recommend an appropriate treatment 6, 7. In standard clinical practice, some patients log migraine attacks and/or days using paper or electronic diaries, calendars, spreadsheets, or documents 8. The primary feature tracked with these methods is the number of days with migraine. However, information on related factors such as potential triggers, pain severity, or functional impact could help the patient and provider better understand the patient’s migraine attacks. In this context, there is an increasing interest among providers and patients to utilize digital solutions capable of capturing and visualizing tracked data 9-15. A recent work from van de Graaf reviewed digital health tools that have been used in headache studies and concluded that electronic headache diaries are useful tools for informing treatment 13. Nevertheless, they identified learnability and ease of use as areas needing improvement among these digital health tools 13.
VEGA is a smartphone application (app) for logging and detailing migraine attacks, potential triggers, and medication usage concurrent with the attack. It is capable of producing analytics and reports for the participant and their healthcare provider. This app aims to help better understand migraine and related treatment decisions/patterns and facilitate patient-provider communication.
The objective of this human factors study was to evaluate the experience of individuals with migraine using the VEGA app, specifically for logging migraine attack characteristics and related medication use. Our hypothesis was that the VEGA app would be rated as “easy to use” and be considered to provide accurate patient-specific data. To test this hypothesis, a group of patients with migraine used the VEGA app for 4 weeks and answered a series of questions about their experience and satisfaction with the app at the end of that 4 weeks period.
Methods
Study Population
Participants resided in the United States and were 18 years or older, able to read/speak English, and owned an iPhone 7 or newer with iOS 12 or above (at the time of the study iPhone was the only device that supported the VEGA app). Neither the participants nor their families were employees of a biotechnology or pharmaceutical company. All participants consented for their data to be used for research purposes. Data from the study were not shared with any other organization. All individuals self-reported a medical diagnosis of migraine and reported experiencing 6 to 20 days of migraine headache per month for the last 3 months. In addition, all participants reported current use of an acute and/or preventive prescription medication for migraine and were under the care of a healthcare provider.
VEGA App
This version of the VEGA app was customized for a short-term research study. Participants were provided with usernames, passwords, and a quick start guide with instructions for the app onboarding process, trigger setup, and an overview of app core functionality. During app onboarding, participants viewed a tour of app features and first-time-use tooltips upon initial visit to certain screens. Within the app, the onboarding tour could be referenced through the settings menu, along with frequently asked questions and other informational content.
In this version of the VEGA app, participants were able to add migraine triggers (e.g., activity, menstruation, and weather) via passive and active measures. Participants could also add potential migraine triggers including alcohol, caffeine, or chocolate consumption, eye strain, fatigue, or stress (Figure 1). In addition, they could enable Global Positioning System location, weather retrieval, and opt in to sync physical activity and menstruation cycle detail from Apple Health. Users could enter data for chosen active measures in the app as often as needed.
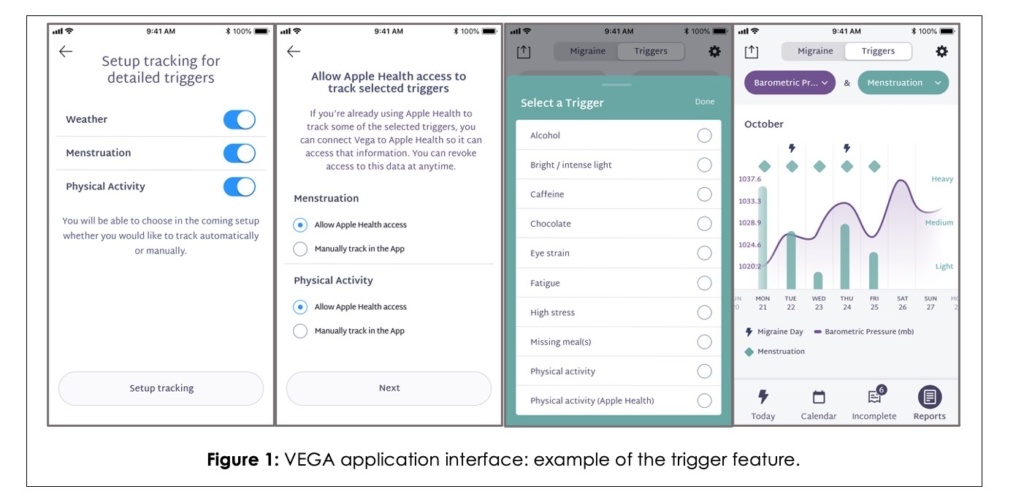
The app provided multiple methods for logging migraine day details and medication use for the migraine attack, such as buttons on the main screen, ability to navigate to previous dates for historical entries through the calendar screen, an optional Apple Watch interface, optional Apple Siri voice commands, and Apple iOS app widgets. Siri shortcuts allow the patient to speak a command to log their migraine-related data without looking at the phone or opening the app. Data that was not logged with minimally acceptable details would appear in the “incomplete” screen for later reference and follow-up.
Within the report functionality in the VEGA app, participants were able to generate a Portable Document Format (PDF) file visualizing data logged for the most recent 90-day period, which could be shared with a healthcare provider digitally or with a printed copy. Participants could also view, explore, and compare visualizations for previously entered data for triggers and migraine days.
Outcomes
After 4 weeks of logging data, the VEGA app-generated a migraine report containing 4 weeks of data that participants could share with their provider through the app. The report was collected between March 06, 2020, and June 03, 2020. It summarized migraine attack characteristics, including functional impact and pain severity, and medication use. At the end of the study, participants were also asked to respond to a survey about their experience and satisfaction, collected between March 17, 2020, and April 25, 2020. The ease of use of the app and the participant’s satisfaction were scored using a Likert scale of 1−6 (with 6 being easiest or most satisfied).
Statistical Analyses
Descriptive analyses were conducted on the study population and results for the survey and app-generated data were summarized as frequencies or percentages.
Results
Baseline characteristics
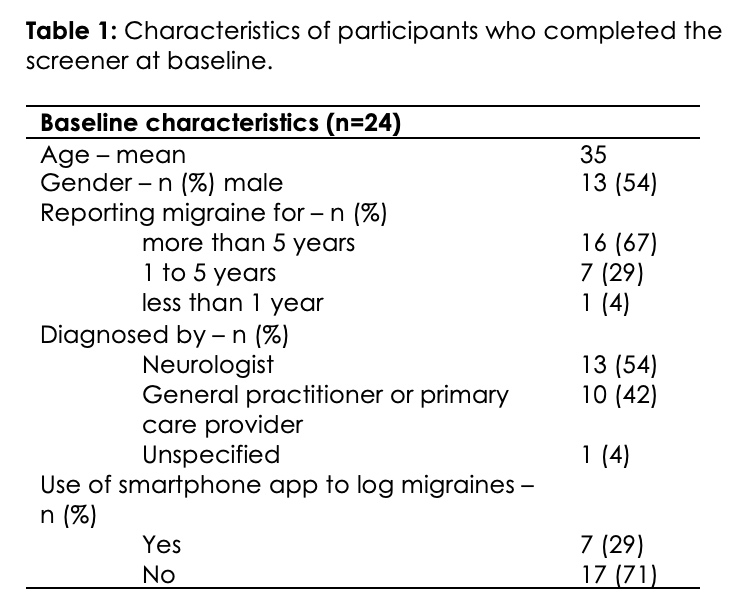
A total of 24 participants completed the screener. Within the 3 months prior to the start of the study, all participants reported at least 6 days with migraine per month. More precisely, 10 participants (42%) reported 6 to 10 monthly migraine headache days, 10 participants (42%) reported 11 to 15 monthly migraine headache days, and 4 participants (16%) had 16 to 20 monthly migraine headache days.
Twenty-two participants (92%) completed the study, while 2 participants logged in once without logging any migraine days or medications during the study period and did not complete the follow-up survey. The baseline characteristics of participants are summarized in Table 1. All participants were diagnosed with migraine by a healthcare provider and 67% (n=16) reported a history of migraine for more than 5 years (Table 1). None of the participants were fully satisfied with their migraine treatment.
Migraine Report
Among the 22 participants who completed the study, 4 (18%) did not share their migraine report with their healthcare provider, whereas 18 participants shared their migraine report, logging a total of 160 migraine days. Over the course of the study, the average migraine days was 9 per participant. In total, participants reported the functional impact of migraine for 96 of those days as follow: 2 days with mild impact, 44 days with moderate impact, 50 days with severe impact (Figure 2A). No details regarding functional impact were provided for 64 of the total migraine days logged in the VEGA app (Figure 2A).
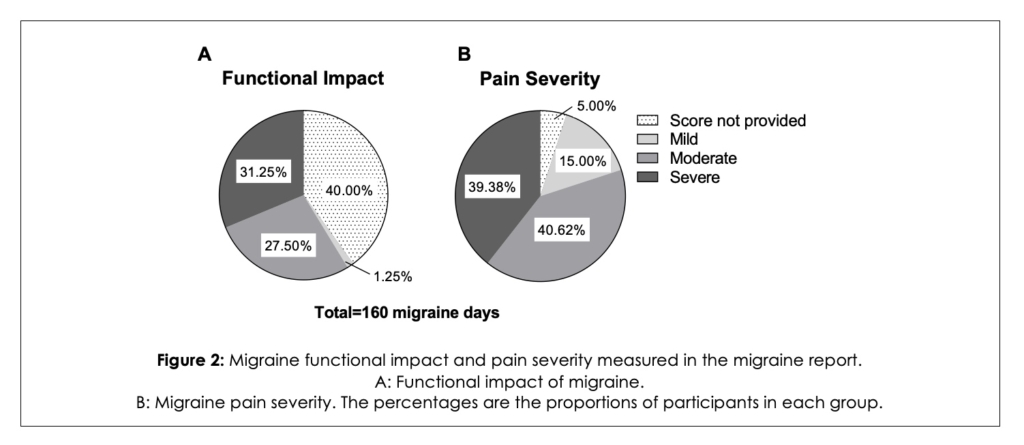
Participants could also rate the pain severity for each of the 160 migraine days. The pain was rated as mild for 24 days, moderate for 65 days, and severe for 63 days and 8 migraine days did not include pain severity details (Figure 2B).
Over the course of the study, all participants recorded a total of 304 doses of medication. Of the acute analgesic medications logged, 36 were prescription and 122 were over the counter. A total of 146 doses of preventive medications were taken.
Participant Survey
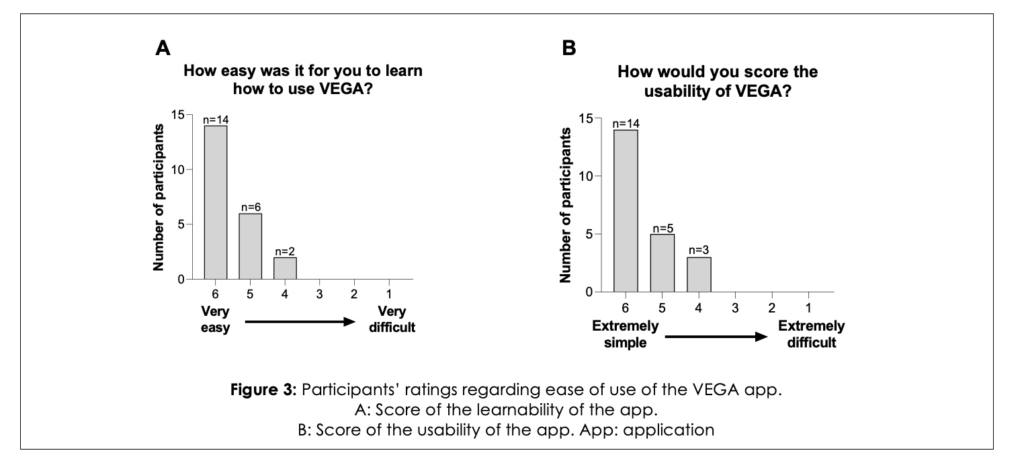
A total of 22 participants completed the survey and all reported they found the VEGA app easy to use. Regarding the ease of learning to use the app, 14 participants reported learnability as “very easy” and 6 reported it as “easy” (Figure 3A). Also, 14 participants found the VEGA app’s usability “extremely simple” and 5 participants reporting it to be “simple” (Figure 3B).
All participants filled out their medication list during setup. Although 12 (54%) participants found the medication feature “very useful”, 5 (22%) participants reported not being able to find their medications listed in this version of the VEGA app. Only 3 (14%) participants set up Siri shortcuts during installation.
While 2 (9%) participants were not familiar with migraine triggers prior to downloading the app, 20 (91%) participants set up the trigger logging feature
in the app. Among those, 7 participants (35%) found the trigger feature “extremely simple”, and 8 (40%) participants found it “simple” to use. Twenty (91%) participants set up automated weather tracking, and 16 (80%) found it useful. Seventy percent of the 20 participants logged their physical activity in the VEGA app. In addition, 7 (35%) female participants integrated their menstruation details with Apple Health. Among the 17 participants who used the trigger graph feature in the app, 8 (47%) of the users graded it as “very useful” or “useful.”
Over 22 participants who completed the study, only 7 (32%) participants generated a PDF healthcare provider report. Among them, 5 (71%) participants graded the report as being “very helpful,” and 1 (14%) participant shared it with their healthcare provider.
Finally, every day of the week (throughout the day and evening), headache data was logged by 1 or more of the participants with the most engagement observed on Thursdays and at 7:00 pm for the full study duration.
Discussion
The objective of this human factors study was to evaluate the experience of individuals with migraine using the VEGA app and how they interacted with the app to log migraine characteristics and medication usage. In this 4-week study, participants were able to successfully download and set up the VEGA app and consistently engaged with it by entering migraine days and characteristics, including medication use, migraine symptoms, and associated triggers. In addition, most participants logged at least 1 migraine day and completed a migraine report. The majority of migraine days were recorded as having a moderate-to-severe functional impact and pain level. Of note, the characteristics of only 5% of all migraine days were not provided, illustrating a high level of engagement with the VEGA app.
Interest in using digital solutions for logging headache is growing, a recent review highlighted an opportunity for improving the learnability and ease of use of these tools 13. While recording migraine data during an attack may not be feasible in all situations, the intent of the VEGA app is to make it easy to collect data concurrent with the attack. The data collected indicate that the participants considered the VEGA app easy to learn and simple to use. This may result in the participant believing the VEGA app participant experience is intuitive and not burdensome such that it could be a useful app for people with migraine to log their disease characteristics.
Although the primary objective of the current study, to assess the usability of the VEGA app, was achieved, certain limitations must be acknowledged. The number of participants was low, and the number of participants was almost evenly split among men and women. Given that migraine is more prevalent in women than men 1, it is possible that the migraine attack and medication use reported among those participants may not be reflective of all individuals with migraine. However, given that men and women had a similar experience regarding learning and using the VEGA app, a change in the proportion of women to men would be unlikely to change the participant ratings. In addition, the VEGA app was not validated using a paper diary or by other means. Certain characteristics related to the attack (e.g., pain duration, some associated symptoms) were not available to log and could have limited the utility of the app for some participants. Some participants were not able to log all the medications they used, as they were not available in the medication feature of the app at the time of the study. Moreover, VEGA was only available for iPhone devices at the time of the study, since then the VEGA app has also become available to those with an Android mobile device.
Electronically logging migraine attack features such as pain severity and functional impact, and medication use could allow patients better insight into the functional burden and characteristics of their attacks. Possibilities for VEGA updates include providing feedback to patients, including customization of notifications, nudges or reminders for each user, or identification of potential patterns in triggers, symptoms, or onset of migraine. This information could help healthcare providers be better equipped to provide the appropriate treatment needed.
Conclusion
This human factors study demonstrated consistent ease of use of the VEGA app in generating patient-reported data surrounding a migraine attack. Future studies should be aimed at assessing broader applicable endpoints and enroll a larger cohort of participants with demographics and migraine frequencies that better represent the overall population of people with migraine.
Acknowledgements
We gratefully acknowledge the contribution and dedication of all the patients who participated to the study, and their family, as well as the extended VEGA product team and cross-functional stakeholders. We also thank Jianyu Chai (former contractor for Eli Lilly and Company) who contributed to the early stages of the study and has no conflict of interest. We also thank James Kershner (former employee of Eli Lilly and Company) for his work on the human factors study. Finally, we thank Marina Schverer, PhD (employee of Eli Lilly and Company) for providing assistance in writing this manuscript.
References
- Collaborators GBDH. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. Nov 2018;17(11):954-976. PubMed PMID: 30353868; PubMed Central PMCID: PMCPMC6191530. doi:10.1016/S1474-4422(18)30322-3
- Digre KB. What’s New in the Treatment of Migraine? J Neuroophthalmol. Sep 2019;39(3):352-359. PubMed PMID: 31393282. doi:10.1097/WNO.0000000000000837
- Katsarava Z, Mania M, Lampl C, Herberhold J, Steiner TJ. Poor medical care for people with migraine in Europe – evidence from the Eurolight study. J Headache Pain. Feb 1 2018;19(1):10. PubMed PMID: 29392600; PubMed Central PMCID: PMCPMC5794675. doi:10.1186/s10194-018-0839-1
- Robbins MS. Diagnosis and Management of Headache: A Review. JAMA. May 11 2021;325(18):1874-1885. PubMed PMID: 33974014. doi:10.1001/jama.2021.1640
- Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. Oct 2000;88(1):41-52. PubMed PMID: 11098098. doi:10.1016/s0304-3959(00)00305-5
- Lipton RB, Stewart WF. Acute Migraine Therapy: Do Doctors Understand What Patients With Migraine Want From Therapy? Headache: The Journal of Head and Face Pain. 1999;39(s2):S20-S26. doi:10.1111/j.1526-4610.1999.00006.x
- Lipton RB, Hahn SR, Cady RK, et al. In-office discussions of migraine: results from the American Migraine Communication Study. J Gen Intern Med. Aug 2008;23(8):1145-51. PubMed PMID: 18459012; PubMed Central PMCID: PMCPMC2517978. doi:10.1007/s11606-008-0591-3
- Giffin NJ, Lipton RB, Silberstein SD, Olesen J, Goadsby PJ. The migraine postdrome: An electronic diary study. Neurology. Jul 19 2016;87(3):309-13. PubMed PMID: 27335112; PubMed Central PMCID: PMCPMC4955275. doi:10.1212/WNL.0000000000002789
- Stubberud A, Linde M. Digital Technology and Mobile Health in Behavioral Migraine Therapy: a Narrative Review. Curr Pain Headache Rep. Jul 31 2018;22(10):66. PubMed PMID: 30066141. doi:10.1007/s11916-018-0718-0
- Santoro A, Delussi M, Leone M, et al. Effects of Botulinum Toxin on Migraine Attack Features in Chronic Migraine: A Six-Month Open-Label Observation Study through Electronic Diary Smartphone Application. Toxins (Basel). Nov 15 2019;11(11)PubMed PMID: 31731628; PubMed Central PMCID: PMCPMC6891747. doi:10.3390/toxins11110668
- Miller VE, Faurot KR, Palssson OS, et al. Comparing prospective headache diary and retrospective four-week headache questionnaire over 20 weeks: Secondary data analysis from a randomized controlled trial. Cephalalgia. Nov 2020;40(13):1523-1531. PubMed PMID: 32799667. doi:10.1177/0333102420949180
- Minen MT, Adhikari S, Padikkala J, et al. Smartphone-Delivered Progressive Muscle Relaxation for the Treatment of Migraine in Primary Care: A Randomized Controlled Trial. Headache. Nov 2020;60(10):2232-2246. PubMed PMID: 33200413. doi:10.1111/head.14010
- van de Graaf DL, Schoonman GG, Habibovic M, Pauws SC. Towards eHealth to support the health journey of headache patients: a scoping review. J Neurol. Jun 11 2020;PubMed PMID: 32529582. doi:10.1007/s00415-020-09981-3
- van Casteren DS, Verhagen IE, Boer I, et al. E-diary use in clinical headache practice: A prospective observational study. Cephalalgia. May 2 2021:3331024211010306. PubMed PMID: 33938248. doi:10.1177/03331024211010306
- Filipi JM, Khairat S. Tracking and visualizing chronic headache trends through the use of linked mobile and desktop websites. Stud Health Technol Inform. 2013;190:45-7. PubMed PMID: 23823370.
Declarations/Disclosures
Funding:
This study was funded by Eli Lilly and Company.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Financial relationships: Bert B. Vargas, Lauren Madar, Jeanine Brosch, William R. Prucka, and Robert A. Nicholson are employees of Eli Lilly and Company and may own stock in this company. Katherine J. Selzler is a minor shareholder and a former employee of Eli Lilly and Company. Rahul Singhal has no conflict of interest. The VEGA application was created by Eli Lilly and Company. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
