Author: Mark Stillman * MD 1, Fetnat Fouad-Tarazi * MD 2, Lan Zhou MD, PhD 1,Robert Shields MD 1, Nicolas Thompson MS 3, Fredrick Jaeger DO 2, Kenneth Mayuga MD 2, Neil Cherian MD 1, Matthew Karafa PhD 3, Katherine Butters CNMT 2, Mary Horvat CRC 1
Author Affiliation:
1 Department of Neurology, Cleveland Clinic Foundation, Cleveland, OH, USA
2 Department of Cardiology, Cardiovascular Institute, Cleveland Clinic Foundation, Cleveland, OH, USA
3 Department of Quantitative Health Sciences, Cleveland Clinic Foundation, Cleveland, OH, USA
* These authors contributed equally to the manuscript.
Competing Interests: The author/s declare no competing interests.
Issue: 06.01
DOI: 10.30756/ahmj.2021.06.01
Received: Sept 29, 2021
Revised: Oct 22, 2021
Accepted: Oct 30, 2021
Published: Nov 16, 2021
Recommended Citation: Stillman M, Fouad-Tarazi F, Zhou L, Shields R, Thompson N, Jaeger F, Mayuga K, Cherian N, Karafa M, Butters K, Horvat M. Autonomic Dysfunction among Migraineurs with and without Complaints of Orthostatic Intolerance: Evidence for Small Fiber Nerve Damage. Ann Head Med. 2021;06:01. DOI: 10.30756/ahmj.2021.06.01
Background and Objective: Migraineurs often complain of orthostatic intolerance (OI), and its recognition is vital to appropriate treatment. This study attempts to identify and characterize autonomic dysfunction, comparing a sample of migraineurs with OI with a sample of migraineurs without OI.
Methods: In a prospective cohort study, we examined one migraine sample complaining of OI for > 6 months (Group 1) and another group without OI (Group 2), using a 70-degree, 45-minute head-up passive tilt-table (HUT45) test, electrocardiographic R-R interval measurements during deep breathing, blood pressure and heart rate monitoring during Valsalva and release, QSART, and skin biopsy for nerve fiber density. We compared results with standard, 10-minute head-up passive tilt-table (HUT10) results to determine which test promoted greater sensitivity, specificity and diagnostic accuracy.
Descriptive statistics were computed for each group, defined by the presence or absence of OI. We conducted several receiver operating characteristic analyses to determine whether certain clinical characteristics were predictive of symptoms of OI.
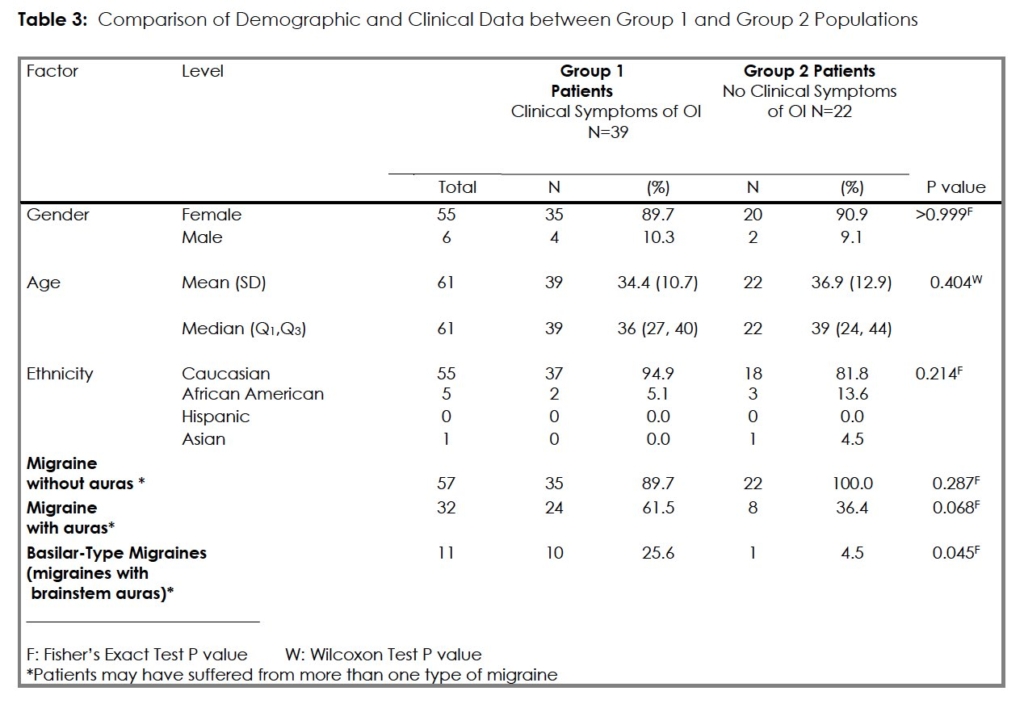
Results: Thirty-nine Group 1 and 22 Group 2 subjects were studied and were similar demographically. Thirty eight of 39 (97.4%) Group 1, versus 15/22 (68.2%) Group 2 subjects, manifested abnormal HUT45 results, a significant difference (p=0.002). The HUT45 showed higher sensitivity (0.59; 95% CI: 0.42-0.74) than the standard 10-minute tilt-table test (HUT10) for all expressions of OI (0.31; 95% CI: 0.17-0.48). No differences in autonomic laboratory or skin biopsy findings distinguished the groups. We detected biopsy-proven small fiber neuropathy in 22/39 (56.4%) Group 1 and 10/22 (45.5%) Group 2 subjects.
Discussion and Conclusions: In migraineurs, OI is an expression of autonomic dysfunction. We found evidence for a peripheral autonomic deficit on skin biopsy in 45% or more of both groups of migraineurs. Extending the head-up tilt table test to 45-minutes increases diagnostic sensitivity, spares patients misdiagnosis and unnecessary testing, and satisfies the principle of ecological validity.
Abbreviations
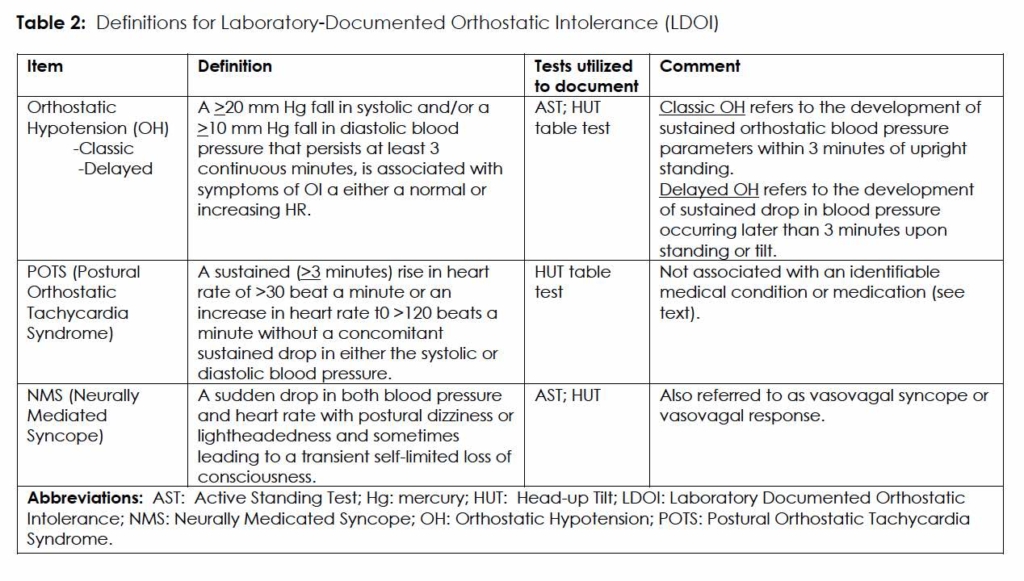
AAN: American Academy of Neurology; AST: Active Standing Test; CNS: central nervous system; ECG: electrocardiograph; IENFD: intra-epidermal nerve fiber density; HUT: passive head-up tilt at > 60 degree incline; HUT45: HUT table test for full 45-minutes: HUT10: HUT table test for the first 10 minutes ; HUT5: HUT table test for the first 5 minutes ; LDOI: laboratory-documented orthostatic intolerance; NMS: neurally mediated syncope; OI: orthostatic intolerance; OH: orthostatic hypotension; PNS: peripheral nervous system; POTS: postural orthostatic tachycardia syndrome; QSART: quantitative sudomotor axon reflex test; SFN: small fiber neuropathy. TAC: trigeminal autonomic cephalalgia
Introduction
Among the primary headache disorders, trigeminal autonomic cephalalgias (TACs) and migraine headaches are associated with autonomic symptoms and signs.1, 2 While TACs are operationally defined by focal autonomic signs and symptoms, among migraineurs these focal signs occur but are less common.2 With migraine headaches, autonomic dysfunction may present more systemically as orthostatic intolerance (OI) – dizziness or lightheadedness, weakness, near syncope or syncope – and lead to considerable physical and psychological morbidity. The spectrum of OI, including orthostatic hypotension (OH), postural tachycardia syndrome (POTS), and syncope (neurally mediated syncope; NMS),3 frequently goes unrecognized or misdiagnosed. Unsuspecting clinicians may label patients – to their patients’ detriment – hysterical, anxious, panic-stricken, or malingerers, or just characterize the event as a ‘syncopal aura.’
Orthostatic intolerance in migraineurs has attracted the attention of researchers since the 1960’s. 4-15 It has been attributed to the peripheral nervous system (PNS) or the central nervous system (CNS), or both. In the general neurology population, one cause of OI, pandysautonomia, results from PNS dysfunction due to damage to small poorly myelinated nerve fibers, including autonomic nerve fibers, 3, 16 and new information has emerged on the presence of autoantibodies directed at ganglionic cholinergic and noradrenergic receptors causing peripheral neuropathic forms of orthostatic intolerance. 4, 17 OI can also be attributed to any of a variety of neurological disorders of the CNS, such as neurodegenerative diseases, 8 and factors such as blood loss, hypovolemia, or volume dysregulation may contribute to the symptoms of the posturally challenged, dizzy, tachycardic migraineur. 18 The cause may also be drug related or idiopathic. 3 To date, no answer exists as to the etiology of OI in the migraine population, and clinical therapeutic approaches to this problem remain limited as a result.
The goal of this study is to identify the presence of, and to clinically characterize, orthostatic intolerance among migraineurs. We investigated this with the battery of tests available in our hospital autonomic laboratory, including the head-up tilt table (HUT) test. To this we added skin biopsy for intra-epidermal nerve fiber density (IENFD) analysis in order to detect peripheral autonomic dysfunction among migraine sufferers with or without auras. 19 We were particularly interested in determining whether the standard HUT, as recommended by European and American autonomic societies, 15 was sensitive enough to detect OI in populations of migraineurs who present to clinic with postural complaints, and whether the accepted standard of a 10-minute 60–80-degree passive, head-up tilt satisfied the principle of ecological validity. 20
For purposes of this study, OI is defined by the presence of any combination of the following symptoms that arise when a patient assumes an upright posture, and remit with assuming a supine posture: palpitations and rapid heartbeat, chest discomfort, shortness of breath, fatigability, exercise intolerance, lightheadedness, pre-syncope and syncope, inattention, anxiety, visual fading or concentric constriction, acrocyanosis and livedo reticularis, and lower extremity weakness. 15 In adults aged 19 or older, postural tachycardia syndrome (POTS), a common form of OI, refers to the development of dizziness, with or without syncope, accompanied by a sustained increase in heart rate from baseline of greater than 30 beats per minutes (or a sustained heart rate of >120 bpm), when assuming an upright posture. 15
Patient Samples
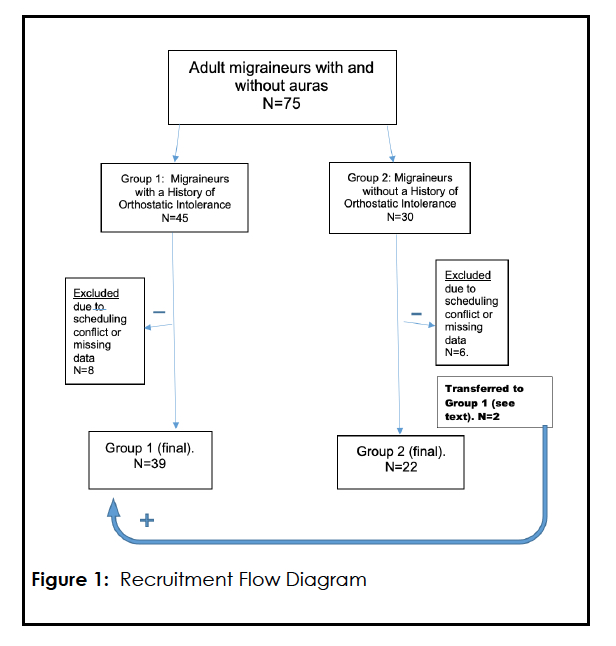
Using a prospective cohort design, we studied two samples of migraineurs – one with symptoms of OI (Group 1) and another without symptoms of OI (Group 2). Between January 2008 and December 2011, adults, 18 years or older, were recruited from patients referred to one practitioner’s (MS) headache practice. This clinician queried every study subject using common language for each of the symptoms mentioned above, and patients were offered admission to the study only if they suffered from migraines with and/or without auras, as defined by the International Classification of Headache Disorders, 2nd edition. 21 Patients were consecutively recruited to either the first or second group as described below. All patients studied had to meet admission criteria.
In Group 1, we studied ambulatory migraine patients with or without auras who had symptoms of OI of >six months duration and had the daily occurrence of at least two symptoms of OI. Symptoms had to remit or significantly improve with assumption of the supine posture. The patients could not be taking any medications known to induce orthostasis: diuretics, alpha blockers and other anti-hypertensive agents, carbonic anhydrase inhibitors including topiramate and zonisamide, anti-Parkinson drugs, phenothiazines and other dopamine blockers, tricyclic antidepressants, norepinephrine transport blockers (e.g., bupropion), vasoconstrictors and decongestants. Patients underwent extensive history and physical examination, including a review of previous medical records, as a screen for any medical condition that could cause orthostasis or autonomic dysfunction including, but not limited to diabetes, thyroid disorders, pheochromocytoma, adrenal insufficiency, cardiac disease, drug or alcohol abuse, degenerative neurological diseases, heavy metal and chemotherapeutic agents toxicity, volume depletion or active gastrointestinal bleeding, or any clinically evident neuropathic condition. Likewise, specific questioning reviewed family histories for neuropathic diseases. In each case, as part of the initial work-up, all patients underwent the Active Standing Test (AST) to screen for initial orthostatic hypotension and classic orthostatic hypotension. 22 All patients studied were headache-free for five or more days and no patients were bedbound.
Group 2, a cohort of adult patients suffering from migraines with or without auras, but who did not possess current or remote histories of OI, was recruited and studied in a fashion identical to Group 1. This group, consisting of otherwise healthy migraineurs was consecutively recruited from the same single headache practice and was subjected to the same limitations on medications and medical conditions, described above. Patient data were included for evaluation only if there were complete data from skin biopsy analysis and a 45-minute passive head-up tilt table test (HUT45) performed in the Cardiology Syncope Clinic Laboratory.
In Group 1 eight subjects (all female, mean age 32.1; median age 33.0) were dropped from evaluation due to missing HUT45 (N=6) or skin biopsy data (N=2). A total of eight subjects in Group 2 who initially agreed to be studied were either withdrawn due to scheduling conflicts (N=6; 5 females, 1 male; mean age 33.6 and median age 32.5) or were transferred to Group 1 (N= 2). These two subjects admitted after study initiation that they had suffered from orthostatic dizziness, presyncope and even fainting as children and were transferred to Group 1. Figure 1
The sample size was based on the available data of subjects recruited and meeting inclusion criteria during the study period. No formal matching method was done for the two groups, but we compared patient characteristics to confirm demographically similarity. A statistical power calculation was not conducted.
Protocol
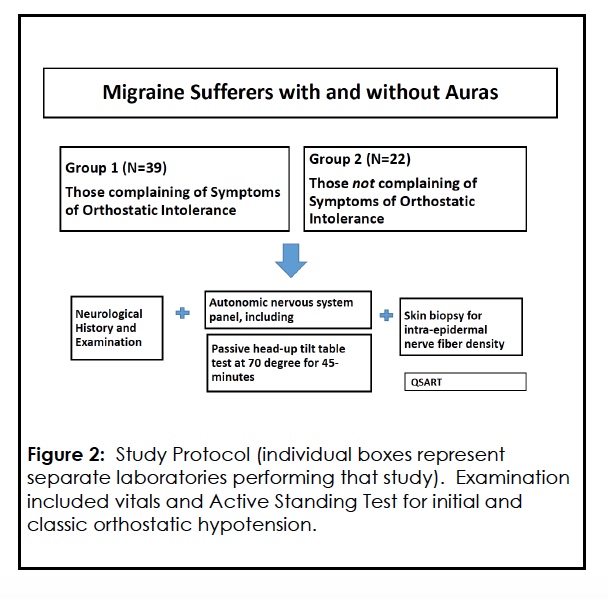
Each patient underwent the following work-up as part of the study. Figure 2
Procedures And Methods
Clinical autonomic testing
Clinical autonomic testing included R-R interval measurements during deep breathing, blood pressure and heart rate monitoring during Valsalva maneuver and release, blood pressure and pulse monitoring during HUT, 3, 23, 24 and Quantitative Sudomotor Axon Reflex Testing (QSART).
Autonomic cardiovascular reflex testing was conducted in the Neurology Department’s Neuromuscular Clinic (RS) utilizing previously published procedures and techniques. 3, 25, 26 Results were compared to normal age-stratified values for these tests and methods published in the literature. 25, 26
Clinical autonomic testing noninvasively evaluated cardio-sympathetic and cardiovagal function.3, 9, 22, 24 Specifically, continuous beat-to-beat heart ratio (R-R interval ratio) and blood pressure monitoring during deep breathing and Valsalva maneuver tested combined sympathetic and parasympathetic function. 9 Evaluation of cardiovascular adrenergic function was assessed by the addition of the above measures during head-upright tilt testing. 3
QSART was performed in the Neurology Department Neuromuscular Clinic (RS) using standard methods published elsewhere. 26, 27 QSART was utilized as a measure of the function of post-ganglionic sympathetic cholinergic nerve fibers that innervate sweat glands. 27, 28 If postganglionic sudomotor nerve fibers are functionally intact, applied acetylcholine binding to the sweat gland muscarinic receptors on eccrine sweat glands will evoke a sweat response. A normal QSART response implies a normally functioning postganglionic sympathetic sudomotor axon and sweat gland. QSART is normal in central and preganglionic autonomic disorders with the caveat that preganglionic autonomic disorders, when chronic, may delay or reduce the QSART response, suggesting trans-synaptic degeneration. 27, 28 Criterion for the presence of a generalized autonomic neuropathy in this study was a reduced or absent QSART response at two or more sites or a reduced or absent response at the proximal foot site.28
Passive 70-degree Head-Up Tilt-table test over 45-minutes (HUT45)
The graded head-up tilt test, standardized in our institution’s Cardiology Syncope Clinic Laboratory (F F-T, FJ, KM, KB), was used for all patients. 23 After obtaining peripheral intravenous access in the arm, patients rested in the supine position in a quiet climate and light-controlled laboratory for at least 20 minutes. A twelve lead ECG was recorded and reviewed at baseline. Blood pressure using a Dinamap automated blood pressure cuff (Midmark Corporation, Miamisburg, OH) as well as manual auscultation and heart rate/ heart rhythm monitoring – using ECG lead II (or best lead)- were obtained over 3 minutes prior to initiation of graded head-up tilt positioning. If vital signs were unstable, the heart rate and blood pressure were monitored until stabilization before study initiation.
Throughout the study (baseline, graded HUT, and recovery), systolic blood pressure, diastolic blood pressure and heart rate were recorded at one-minute intervals using an automated blood pressure cuff. In addition, heart rate and rhythm were monitored continuously using ECG waveforms. Patient symptoms were noted and registered during the entirety of the study. No pharmacological medications were administered to any patient during HUT.
The tilt table was moved sequentially to a 30-degree angle for two minutes, followed by a 45-degree angle for two minutes, and then followed by a 70-dgree angle for 45 minutes. The upright portion of the test could be stopped early at any time based on patient symptoms, patient request, or blood pressure/heart rate changes indicating an abnormal response. At the completion of the upright portion of the test, the tilt table was returned to the supine position for a recovery period of at least five minutes. Twelve lead ECGs were obtained at baseline, during upright tilt, and during recovery, both at set intervals and as needed for symptoms or changes in vital signs or heart rhythm. 18, 23
Passive HUT tests the physiologic response to the gravitational pull of blood volume from the thoracic cavity when upright posture is assumed, resulting in reduction of venous return to the right heart. 16 It is the cornerstone in the assessment of OI, presyncope, and syncope. Considering its widespread availability, the HUT test was the procedure we utilized to assess the clinical manifestations of orthostatic intolerance in our study samples. 16
The above combination of studies, now considered routine in most autonomic laboratories, was used to evaluate both peripheral and central autonomic function. 24
Punch skin biopsy for intraepidermal nerve fiber density (IENFD) analysis
This procedure, performed by the Neurology Department Neuromuscular Clinic (LZ), provides a window to the peripheral nervous system at the level of sweat gland and arteriolar/venular innervation by measuring the extent of sympathetic post-ganglionic nerve fiber density at the histological level. The biopsy procedure and immunohistochemical staining have been described in detail elsewhere. 29-31 IENFD assessment using immunohistochemistry is a validated, reproducible method for the evaluation of small fiber, including autonomic, neuropathies. 30-32 Skin biopsy was selected as the diagnostic standard and was used to validate findings of QSART, the more commonly accessible test in clinical practice.
Categorization of HUT studies
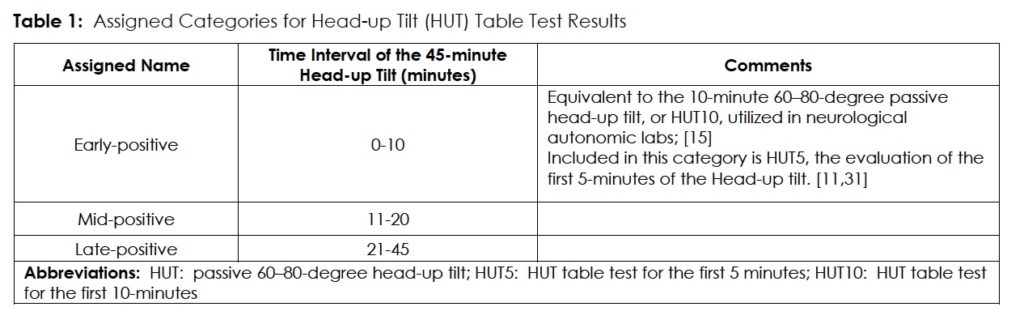
In a post hoc analysis of data, to assess the sensitivity of prolonged, 45-minute HUT table testing, we decided to subdivide HUT table testing results into three subcategories. (Table1) Endpoints for a positive test were the same as mentioned above.
This decision was based on the observation that many patients with orthostatic intolerance-particularly POTS- exhibit abnormal HUT tests when tilted for a full 45-minute, 70-degree tilt in our Cardiology Syncope Clinic Laboratory, as opposed to our Neurology Department Autonomic Laboratory, where a 60-degree tilt is limited to 10-minutes, maximum. The limit of 10-minutes follows the recommendations set forth by the American and European autonomic societies, as stated in the American Academy of Neurology’s consensus statement on the definition of POTS.15 We opted to use our institution’s Cardiology Syncope Clinic Laboratory’s 45-minute HUT45, enabling us to address the data from the first 10-minutes of the 45-minute tilt challenge as a proxy for the standard 10-minute tilt table test.
For analysis, we grouped the results from the ‘mid-positive’ period with the ‘late-positive’ measurement period of the 45-minute HUT and compared the outcomes for POTS, OH, and NMS with first 10 minutes of the subject’s HUT10. In this fashion we used the HUT10 to simulate the standard 10-minute HUT without subjecting the individual patient to two separate procedures. We then evaluated the remainder of the 45-minutes of 70-degree passive tilt as part of our primary objective.
We further subdivided early positives into those who became positive in the first 5-minutes of 70-degree tilt (HUT5), to determine the sensitivity and specificity of this measurement in diagnosing POTS, as mentioned by Low et al.11 and Khurana and Nicholas. 33
In our evaluation of Group 1 and Group 2, abnormal responses to HUT testing were assigned the label laboratory-documented orthostatic intolerance (LDOI) to reflect the clinical spectrum of OI. 3 (Table 2)
Standard Protocol, Approvals, Registrations, and Patient Consents
Approval for this study was obtained from the Cleveland Clinic’s Institutional Review Board. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Freely given, written informed consent was obtained from all participants in the study, including consent to publish anonymized clinical details obtained from the patients, all of which is available for review by the editors of this journal.
Data Availability
Anonymized data will be shared, upon request, with any qualified investigator.
Statistical analysis
Descriptive statistics were computed for each group, defined by presence or absence of clinical symptoms of orthostatic intolerance (OI). The only continuous variable examined was age and was summarized using mean and standard deviation (SD) and median and interquartile range (IQR). Histograms and a Q-Q plot revealed an outlier, so we used the Wilcoxon rank sum test to compare age in the two groups. Categorical variables were summarized with frequency and percentage, and comparisons were made using Fisher’s exact test.
To determine whether certain clinical characteristics were predictive of clinical symptoms of OI, we conducted several different receiver operating characteristic (ROC) analyses. Here, symptoms of OI would be considered the gold standard. For each predictor, we computed the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). The exact binomial method was used to obtain 95% confidence intervals (CI). Additionally, we conducted a Fisher’s exact test to determine whether there was an association between the predictor and clinical symptoms of OI. Predictors examined included HUT5, HUT10, HUT45, LDOI, MHRR, E:I Ratio, Valsalva Ratio, and Blood Pressure Valsalva Respiration Phase 4. The primary analysis for this study was to examine the diagnostic accuracy of HUT45 to predict clinical symptoms of OI. All other ROC analyses were considered secondary. Additional secondary analyses examined laboratory-documented orthostatic intolerance (LDOI) and biopsy-proven small fiber neuropathy (SFN) as gold standard variables. Analyses were conducted using R, version 4.0.3 (Vienna, Austria). All tests were two-sided and P-values < 0.05 were considered statistically significant.
Role Of the Funding Source
The funder of the study had no role in the study design, data collection, data analysis or interpretation or drafting of the paper. The corresponding author had full access to all the data in the study and had ultimate responsibility for the decision to submit for publication.
Results
There were 39 subjects in Group 1, 35 (89.7%) female, and 22 subjects in Group 2 with 20 (90.9%) females. The two groups were clinically and demographically similar. (Table 3) Group 1 had a greater representation of migraine sufferers with auras but this was significant only with respect to migraines with brain stem auras [Group 1 (10/39; 25.6%) compared with Group 2 (1/22; 4.5%); (P=0.045)].
Group 1– Migraineurs with orthostatic intolerance (N=39)
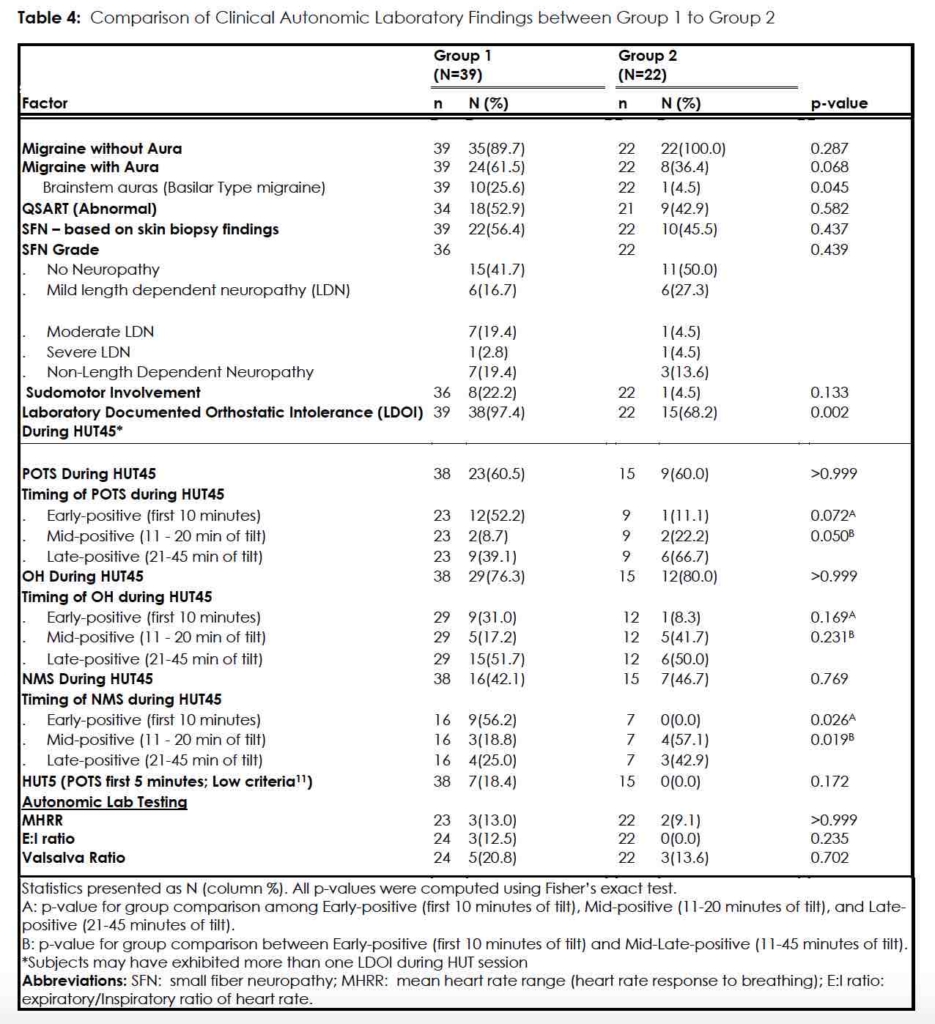
HUT testing (Table 4)
In Group 1 38/39 (97.4%) had a HUT that met defined criteria for LDOI (POTS, OH, and/or NMS; herein referred to as “positive”) within 45 minutes (HUT45). Of these positive responses, 23/38 (60.5%) met POTS criteria and 12/23 (52.2%) tested positive in the first 10 minutes (HUT10, or early-positive), and 7 of these twelve (58.3%) tested positive for POTS in the first five-minutes. Two of the 23 POTS subjects (8.7%) tested positive in the interval between 11 and 20 minutes of 70-degree tilt (mid-positive) while 9/23 (39.1%) tested positive in the last 25 minutes of tilt (late-positive). (Table 4)
With respect to OH 29/38 (76.3%) Group 1 subjects experienced either systolic and/or diastolic hypotension with 9/29 (31.0%) being early-positive, 5/29 (17.2%) being mid-positive, and 15/29 (51.7%) being late-positive. Sixteen subjects (16/38; 42.1%) experienced a neurally mediated syncopal event; 9/16 (56.2%) displayed this in the first 10 minutes of maximal tilt (early-positive) while 3/16 (18.8%) were mid-positive and 4/16 (25.0%) were late-positive. (Table 4)
To summarize, in Group 1, the group of migraineurs symptomatic of orthostatic intolerance, 52.2% of POTS cases, 31.0% of OH cases, and 56.2% of the syncopal events occurred during HUT10, the first ten-minutes of 70-degree tilt. In total, 38/39 (97.4%) Group 1 patients had a LDOI, either in isolation or combination, during the extended 45-minute passive head-up tilt.
QSART (N= 34)
Of the 34 subjects who completed the QSART, 18/34 (52.9%) had an abnormal response. Among these,14/18 (77.8%) patients were skin biopsy positive for small fiber neuropathy and 4/18 (22.2%) were biopsy negative. The remaining 16 of 34 evaluable subjects (47.1%) exhibited normal QSARTs, of which 6 were skin biopsy-positive (6/16; 37.5%), and 10 were biopsy-negative (10/16; 62.5%).
Skin Biopsy for SFN (N=37)
The skin biopsies were positive in 22/39 (56.4%) and negative in 17/39 (43.2%). (Among the five patients who did not have QSART testing, there were two positive and three negative skin biopsies.)
Group 2 – Migraineurs without a history of orthostatic intolerance (N=22)
HUT testing (Table 4)
In this group of migraineurs, 15/22 (68.2%) had a positive HUT45 for POTS, OH, or NMS; 7/22 (31.8%) had unremarkable HUT45’s. Nine of 15 subjects (9/15; 60%) tested positive for POTS, only one of which (1/9; 11.1%) was early-positive; no subject (0.0%) tested positive within the first 5-minutes of 70-degree tilt. Two were mid-positive (2/9; 22.2%) and the remaining six (6/9; 66.7%) were late-positive. Twelve (12/15; 80.0%) Group 2 subjects exhibited OH with 1/12 (8.3%) early-positive, 5/12 (41.7%) mid-positive and 6/12 (50.0%) late-positive. Finally, NMS occurred in 7 Group 2 patients; none experienced syncope in the first 10 minutes and 4/7 (57.1%) events occurred mid-tilt and 3/7 (42.9%) occurred in the last 25 minutes.
In summary, in the Group of migraineurs without OI symptoms, 11.1%, 8.3% and 0%, of POTS, OH, and NMS events, respectively, occurred during standard HUT10. The majority of LDOI events in this group occurred during the extension of HUT past 10-minutes. In total, in Group 2, a LDOI was documented in 15/22 (68.2%) of subjects during the prolonged tilt, the HUT45.
QSART (N= 21)
Of the evaluable Group 2 subjects, 9/21 (42.9%) had an abnormally delayed or absent QSART and 4 of these were biopsy positive (4/9; 44.4%) for small fiber sensory neuropathy while 5 were normal (5/9; 55.6%). The remaining 12 patients (12/21;57.1%) had normal QSARTs, and of these, 8/12 (66.7%) were biopsy negative and 4/12 (33.3%) were biopsy positive.
Skin Biopsy for small fiber neuropathy (N=22)
The skin biopsies in this group demonstrated abnormally low IENFD in 10/22 (45.5%). The remaining 12/22 (54.5%) biopsies were interpreted as normal.
Comparison between Group 1 and Group 2
Clinical history and clinical laboratory evaluation of the two samples of migraine sufferers revealed few measurable differentiating features. Other than a higher prevalence of migraines with brainstem auras in Group 1, the two cohorts were clinically similar. (Table 3) No subject in either group exhibited initial orthostatic hypotension with the AST.
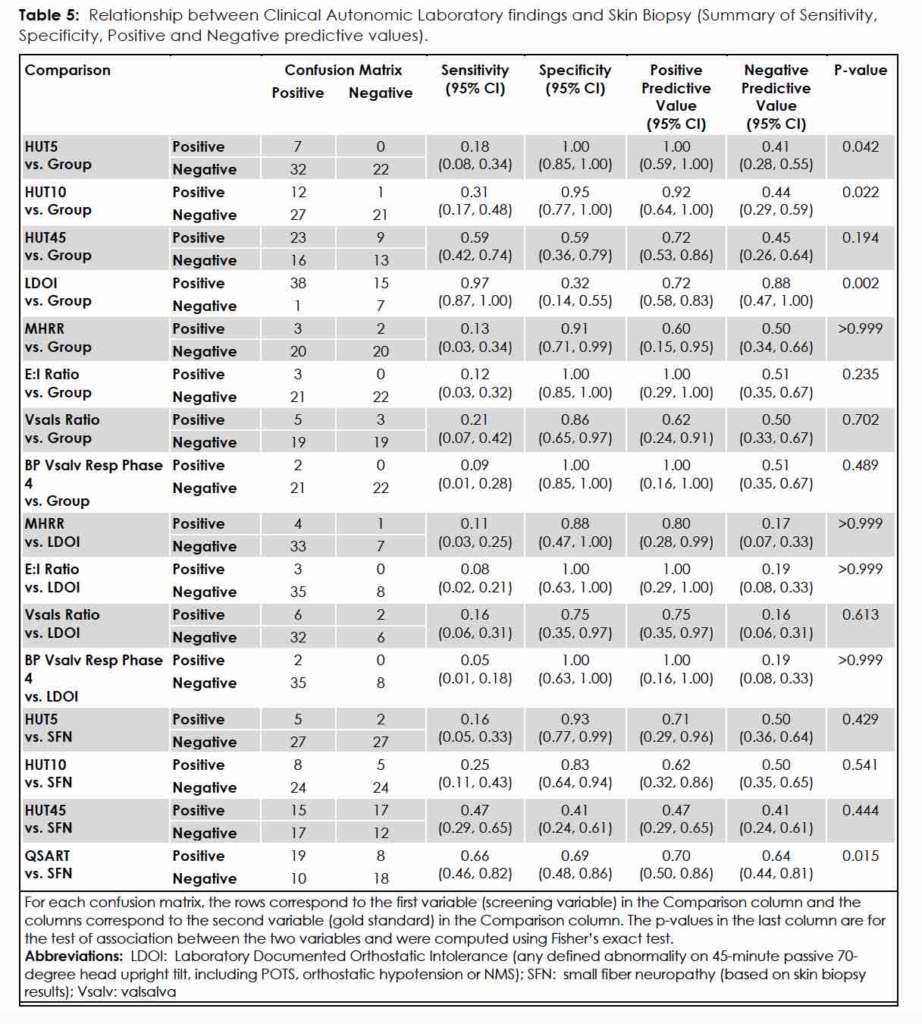
Of the battery of laboratory tests employed, the only measures that separated the subjects in the Group 1 from Group 2 were the Laboratory Documented Orthostatic Intolerance (LDOI) factors determined by HUT45. Thirty-eight of 39 Group 1 subjects (97.4%) registered one or more LDOI factors, in comparison to 15/22 (68.2%) Group 2 subjects (p=0.002). (Table 4) For HUT5 measurement, 7/38 (18.4%) Group 1 subjects versus 0/15 Group 2 subjects (0.0%) were positive, but the difference in percentages was not significant (p=0.172). Specificity for HUT5 measurement was very high (1.00 [95% CI 0.85,1.00]), however, despite a low sensitivity, (0.18 [95% CI 0.08,0.34]) suggesting that the passive head-upright tilt table test, with particular attention to the first five minutes, diagnoses POTS with few false positive results. (Table 5)
Duration of HUT: HUT45 vs HUT10.
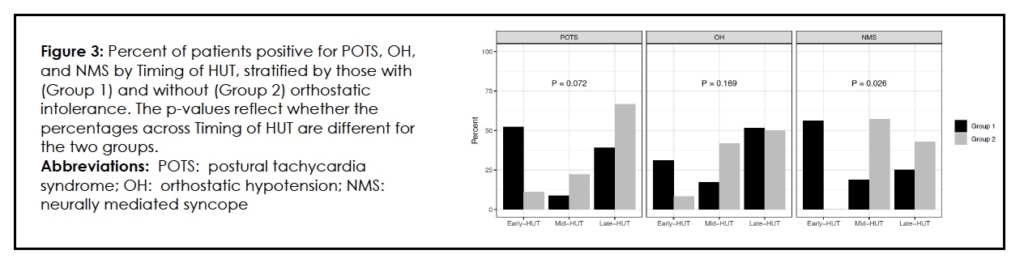
Extending head-up tilt testing to 45 minutes helped segregate Group 1 from Group 2 when each LDOI was reviewed. When comparing the HUT10 with HUT45, interrogation of the additional 35 minutes of passive 70-degree tilt favored Group 2 in the expression of POTS as compared to Group 1. Eight of nine Group 2 patients (88.9%) met POTS criteria after the initial ten minutes. (Even a significant proportion of Group 1 subjects (11/23; 47.9%) developed POTS after the expiration of the first 10 minutes of tilt.) The p-value for this difference between Group 1 and Group 2 in the timing of the expression of POTS during HUT is at the nominal threshold for significance (p=0.050) (Table 2). Conversely, the early registration of POTS (in the first ten minutes of tilt) favored Group 1 over Group 2, but the difference was not significant [12/23; (52.2%) vs 1/9 (11.1%); p=0.072]. (Table 4, Figure 3)
For detection of orthostatic hypotension, extending the HUT beyond the standard ten minutes did not distinguish between Group 1 and Group 2 either in the first 10 minutes or the remainder of the HUT45. (Table 4) This was not the case when we looked at NMS. Group 1 diverged from Group 2 during HUT10, with Group 1 subjects suffering syncope early (p=0.026). (Table 4, Figure 3) The HUT45 revealed a significantly greater percentage of NMS occurring in Group 2, migraineurs without complaints of OI, compared with Group 1, in the 35 minutes following HUT10 [7/7 (100 %) vs 7/16 (43.8%); p=0.019]. (Table 4, Figure 3)
If we assume that the provocation and documentation of syncope, orthostatic hypotension or sustained tachycardia during tilt, simulates clinical orthostatic intolerance during life, 20 the HUT45 demonstrated greater sensitivity [0.59 (0.42,0.74)] than the HUT10 [0.31 (0.17,0.48)]. (Table 5) Looked at another way, 12/39 (30.8%) of Group 1 subjects were positive for POTS during the HUT10 as compared with 1/22 (4.5%) Group 2 subjects, a significant difference (p=0.022). HUT5, with its high specificity for POTS, could distinguish a difference between migraineurs with complaints of OI and those without (p=0.042). (Table 5)
Association between clinical autonomic laboratory findings and skin biopsies in Group 1 and Group 2
Aside from HUT testing mentioned above, neither skin biopsy nor clinical autonomic testing results (RR intervals during deep breathing; pulse and blood pressure readings during Valsalva and release) were able to differentiate Group 1 from Group 2. (Table 4, Table 5)
Association between QSART findings and skin biopsy in Group 1 and Group 2
Table 5 summarizes the sensitivity and specificity and positive and negative predictive values of the laboratory evaluation of the two groups and how they fared in comparison. We found that QSART values were significantly associated with the results of skin biopsy and morphometric analysis for IENFD. For all patients in the study, the QSART had a positive predictive value of 0.70 (0.50, 0.86) and a negative predictive value of 0.64 (0.44, 0.81).
Tables
Discussion
Recent studies recognize the phenomenon of orthostatic intolerance, including syncope, in adult and adolescent migraine populations. 2, 8-10 The spread of estimates of the incidence of migraine headaches among patients with OI is extremely wide, and it is difficult to pinpoint an exact number at the present time. 29 However, the converse phenomenon of migraineurs presenting with symptoms of OI may be more common in clinical practice than the literature suggests.
More than 60 years ago, Bickerstaff reported on basilar migraines describing athletic, healthy, adolescent female migraineurs suffering from what was likely vasovagal syncope in the setting of postural tachycardia syndrome. 6 Recent reports highlight the development of POTS, delayed orthostatic hypotension, and NMS in a predominantly pediatric and adolescent female migraine population following a flu-like illness, injury (athletic or otherwise), or surgery. 25, 26, 33-37 In adults, the age of onset of symptomatic OI parallels the age of onset of migraines in a predominantly female population. 36 The European CAMERA study found an increased lifetime prevalence of syncope and OI without evidence of interictal signs of autonomic nervous system failure in migraineurs,12, 13 and the lifetime prevalence of syncope among migraineurs was 41% (45% in females and 32% in males). 12
Some researchers have suggested that migraines represent a central noradrenergic disorder akin to a degenerative disease such as multiple system atrophy.14 We attempted to segregate central from peripheral autonomic dysfunction in our sample populations but could not convincingly invoke causative CNS evidence. Instead, we were surprised to find a high prevalence of pathologically confirmed small fiber neuropathy in both – 56.4% of Group 1 subjects and 45.5% of subjects in Group 2 Table 4. These findings were perplexing and left us with more questions than answers. Incriminating the PNS might explain a tendency for OI in both groups of migraineurs to be expressed with prolonged tilting, especially if OI manifests as a spectrum of the severity of damage. 14 Considering the high prevalence of SFNs in both groups of migraineurs, the tendency for OI may therefore be anchored to the expression of PNS autonomic damage. Further investigation is necessary.
We cannot speculate as to the etiology of the SFN’s as we did not pursue it diagnostically in this study. We likewise did not investigate potential non-neurogenic cause of OI specifically with hemodynamic/blood volume testing, and we cannot discount a cardiovascular or hemodynamic explanation.18 In terms of the possible effect of deconditioning on HUT results, it is unlikely that deconditioning played a significant role in either group of subjects. All subjects were active, ambulatory and none was bed-bound for even a day before recruitment. Other than chronic migraine, no patient suffered from a chronic debilitating illness.
We specifically compared HUT10 to HUT45 performed by our institution’s Cardiology Syncope Clinic Laboratory using POTS, orthostatic hypotension, and neurally mediated syncope as endpoints (e.g., LCOI). The HUT45 exhibited greater sensitivity than the HUT10 in detecting any combination of POTS, OH or NMS, and more accurately reflected experiences in real life. 20
These findings confirmed our hypothesis that a prolonged duration of tilt is necessary for accurate documentation of OI in this study population; the AAN’s original HUT guidelines were designed to detect flagrant OI, found in conditions such as pure autonomic failure and other severe autonomic neuropathies.15 Recent literature has promoted prolonged tilt testing for POTS and delayed orthostatic hypotension. 22, 23, 34 Our finding of ‘mid-’ (11 to 20-minute delay) or ‘late-’ (21 to 45-minute delay) POTS and/or syncope has been realized by other researchers as well. 23, 38 Mayuga et al. commented on this and were able to distinguish early POTS (within 10-minutes of 70-degree tilt) from late POTS (after 10-minutes of 70-degree tilt) on the basis of age, the presence of hypokinetic circulatory indices, and exposure to certain medications, such as angiotensin converting enzyme inhibitors and angiotensin receptor blockers. 23
As a diagnostic test, the HUT swims in a sea of variables, such as the tilt angle, nonuse or use of a footrest, duration of tilt, and use of procedural drugs, and its utility is complicated by differences in interpretation and lack of reference standards.23, 39, 40 The take-away is that the HUT was never designed to be a stand-alone test, done in isolation from a careful clinical evaluation. It achieves greater sensitivity and specificity (overall approximately 91% for diagnosing NMS) in the context of a full history and physical (including AST), a well-appointed laboratory with well trained staff attentive to patients’ symptoms during the tilt, the use of provocative maneuvers (e.g., Valsalva and deep breathing), and the prudent use of pharmacological agents, where indicated. 16, 41 This statement reflects the 1996 recommendations from the American College of Cardiology which suggested extending the duration of HUT testing up to 45-minutes. 42
We recognize limitations of this study. It is a single institution study, and despite best efforts, may have been subject to selection bias. The small size of the study sample may have resulted in power too low to detect significant associations and some of the confidence intervals for measures of diagnostic accuracy are wide. These results should be interpreted with caution and future studies with larger sample sizes are necessary. We did not pursue the etiology of the small fiber neuropathy, something that needs to be done in future investigations. Finally, we did not subject a cohort of normal adults (non-migraineurs without symptoms of OI) to the above protocol, so we cannot comment whether this cohort would also exhibit an abnormal HUT45 or exhibit peripheral autonomic nervous system pathology on skin biopsy.
Nonetheless, the finding of a high prevalence of small fiber autonomic neuropathy in both the symptomatic and asymptomatic group of migraineurs suggests peripheral autonomic nervous system dysfunction in migraineurs, maybe as a prelude to a systemic disorder, such as an autoimmune disease or an unrecognized neurodegenerative disease. We cannot exclude an additional contributory role played by central autonomic nervous system dysfunction, and other investigations such as functional neuroimaging may provide further insight.
Accordingly, we propose that both groups of migraineurs – orthostatically symptomatic and orthostatically asymptomatic – possess the tendency to develop signs of orthostatic intolerance when challenged with passive head-up tilt test. Our study demonstrates that both groups may develop an abnormal response to HUT if tested long enough. Also, during the initial 10-minutes of 70-degree HUT, there is a significant difference in the development of syncope, favoring the migraineurs with OI. We question whether the difference in expression of OI between the two groups merely reflects different trajectories of the same disease process, and the process appears to be associated with a peripheral nervous system disorder, with or without a CNS contribution.
Conclusions
Orthostatic intolerance in migraineurs with and without auras is a manifestation of autonomic nervous system dysfunction. While we could not definitively invoke any specific central nervous system cause, we detected evidence of peripheral nervous system dysfunction in a high proportion of subjects whether or not they complained of OI.
Extending passive head-up tilt table test beyond the traditional 10-minute limit increased sensitivity in demonstrating OI in these patients, spared patients unnecessary testing and misdiagnoses, and also satisfied the principle of ecological validity.
References
- Headache Classification Committee of the International Headache S. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. Jul 2013;33(9):629-808. PubMed PMID: 23771276. doi:10.1177/0333102413485658
- Danno D, Wolf J, Ishizaki K, Kikui S, Yoshikawa H, Takeshima T. Cranial Autonomic Symptoms of Migraine in Japan: Prospective Study of 373 Migraine Patients at a Tertiary Headache Center. Headache. Sep 2020;60(8):1592-1600. PubMed PMID: 32592512. doi:10.1111/head.13888
- Cutsforth-Gregory JK. Postural Tachycardia Syndrome and Neurally Mediated Syncope. Continuum (Minneap Minn). Feb 2020;26(1):93-115. PubMed PMID: 31996624. doi:10.1212/CON.0000000000000818
- Gunning WT, 3rd, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural Orthostatic Tachycardia Syndrome Is Associated With Elevated G-Protein Coupled Receptor Autoantibodies. J Am Heart Assoc. Sep 17 2019;8(18):e013602. PubMed PMID: 31495251; PubMed Central PMCID: PMCPMC6818019. doi:10.1161/JAHA.119.013602
- Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatry. Feb 1960;23:23-32. PubMed PMID: 14444681; PubMed Central PMCID: PMCPMC495326. doi:10.1136/jnnp.23.1.23
- Bickerstaff ER. Impairment of consciousness in migraine. Lancet. Nov 11 1961;2(7211):1057-9. PubMed PMID: 13868977. doi:10.1016/s0140-6736(61)92538-7
- Gotoh F, Komatsumoto S, Araki N, Gomi S. Noradrenergic nervous activity in migraine. Arch Neurol. Sep 1984;41(9):951-5. PubMed PMID: 6477230. doi:10.1001/archneur.1984.04050200057018
- Havanka-Kanniainen H, Tolonen U, Myllyla VV. Autonomic dysfunction in adult migraineurs. Headache. Sep 1986;26(8):425-30. PubMed PMID: 3771211. doi:10.1111/j.1526-4610.1986.hed2608425.x
- Havanka-Kanniainen H. Cardiovascular reflex responses during migraine attack. Headache. Oct 1986;26(9):442-6. PubMed PMID: 3781830. doi:10.1111/j.1526-4610.1986.hed2609442.x
- Boiardi A, Munari L, Milanesi I, Paggetta C, Lamperti E, Bussone G. Impaired cardiovascular reflexes in cluster headache and migraine patients: evidence for an autonomic dysfunction. Headache. Jul 1988;28(6):417-22. PubMed PMID: 3170188. doi:10.1111/j.1526-4610.1988.hed2806417.x
- Low PA, Opfer-Gehrking TL, Textor SC, et al. Postural tachycardia syndrome (POTS). Neurology. Apr 1995;45(4 Suppl 5):S19-25. PubMed PMID: 7746369.
- Thijs RD, Kruit MC, van Buchem MA, Ferrari MD, Launer LJ, van Dijk JG. Syncope in migraine: the population-based CAMERA study. Neurology. Apr 11 2006;66(7):1034-7. PubMed PMID: 16606915. doi:10.1212/01.wnl.0000204186.43597.66
- Kruit MC, Thijs RD, Ferrari MD, Launer LJ, van Buchem MA, van Dijk JG. Syncope and orthostatic intolerance increase risk of brain lesions in migraineurs and controls. Neurology. May 21 2013;80(21):1958-65. PubMed PMID: 23616159; PubMed Central PMCID: PMCPMC3716345. doi:10.1212/WNL.0b013e318293e1c7
- Peroutka SJ. Migraine: a chronic sympathetic nervous system disorder. Headache. Jan 2004;44(1):53-64. PubMed PMID: 14979884. doi:10.1111/j.1526-4610.2004.04011.x
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. Apr 2011;21(2):69-72. PubMed PMID: 21431947. doi:10.1007/s10286-011-0119-5
- Cheshire WP, Jr., Goldstein DS. Autonomic uprising: the tilt table test in autonomic medicine. Clin Auton Res. Apr 2019;29(2):215-230. PubMed PMID: 30838497. doi:10.1007/s10286-019-00598-9
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. Sep 21 2000;343(12):847-55. PubMed PMID: 10995864. doi:10.1056/NEJM200009213431204
- Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med. Mar 1986;104(3):298-303. PubMed PMID: 3511818. doi:10.7326/0003-4819-104-3-298
- Gibbons CH, Wang N, Kim JY, Campagnolo M, Freeman R. Skin Biopsy in Evaluation of Autonomic Disorders. Continuum (Minneap Minn). Feb 2020;26(1):200-212. PubMed PMID: 31996629. doi:10.1212/CON.0000000000000814
- Pavlovic JM, Buse DC, Sollars CM, Haut S, Lipton RB. Trigger factors and premonitory features of migraine attacks: summary of studies. Headache. Nov-Dec 2014;54(10):1670-9. PubMed PMID: 25399858. doi:10.1111/head.12468
- Headache Classification Subcommittee of the International Headache S. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9-160. PubMed PMID: 14979299. doi:10.1111/j.1468-2982.2003.00824.x
- Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness : Consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Clin Auton Res. Jun 2021;31(3):369-384. PubMed PMID: 33740206; PubMed Central PMCID: PMCPMC8184725. doi:10.1007/s10286-020-00738-6
- Mayuga KA, Butters KB, Fouad-Tarazi F. Early versus late postural tachycardia: a re-evaluation of a syndrome. Clin Auton Res. Jun 2008;18(3):155-7. PubMed PMID: 18470476. doi:10.1007/s10286-008-0472-1
- Low PA. Testing the autonomic nervous system. Semin Neurol. Dec 2003;23(4):407-21. PubMed PMID: 15088262. doi:10.1055/s-2004-817725
- Sandroni P, Benarroch EE, Low PA. Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. J Appl Physiol (1985). Oct 1991;71(4):1563-7. PubMed PMID: 1757382. doi:10.1152/jappl.1991.71.4.1563
- Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology. Nov 22 2005;65(10):1533-7. PubMed PMID: 16301478. doi:10.1212/01.wnl.0000184504.13173.ef
- Sletten DM, Kimpinski K, Weigand SD, Low PA. A novel gel based vehicle for the delivery of acetylcholine in quantitative sudomotor axon reflex testing. Auton Neurosci. Oct 5 2009;150(1-2):127-30. PubMed PMID: 19520617; PubMed Central PMCID: PMCPMC2869290. doi:10.1016/j.autneu.2009.05.250
- Sato K. Normal and abnormal sweat gland function. 2nd ed ed. Clinical Autonomic Disorders: Evaluation and Management. Philadelphia: Lippincott-Raven; 1997:97-108.
- Illigens BM, Gibbons CH. Sweat testing to evaluate autonomic function. Clin Auton Res. Apr 2009;19(2):79-87. PubMed PMID: 18989618; PubMed Central PMCID: PMCPMC3046462. doi:10.1007/s10286-008-0506-8
- England JD, Gronseth GS, Franklin G, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. Jan 13 2009;72(2):185-92. PubMed PMID: 19056666. doi:10.1212/01.wnl.0000336370.51010.a1
- McCarthy BG, Hsieh ST, Stocks A, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. Oct 1995;45(10):1848-55. PubMed PMID: 7477980. doi:10.1212/wnl.45.10.1848
- McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. Dec 1998;55(12):1513-20. PubMed PMID: 9865794. doi:10.1001/archneur.55.12.1513
- Khurana RK, Nicholas EM. Head-up tilt table test: how far and how long? Clin Auton Res. Dec 1996;6(6):335-41. PubMed PMID: 8985622. doi:10.1007/BF02556304
- Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. Mar 2007;82(3):308-13. PubMed PMID: 17352367. doi:10.4065/82.3.308
- Ojha A, Chelimsky TC, Chelimsky G. Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr. Jan 2011;158(1):20-3. PubMed PMID: 20723911. doi:10.1016/j.jpeds.2010.07.005
- Bryarly M, Phillips LT, Fu Q, Vernino S, Levine BD. Postural Orthostatic Tachycardia Syndrome: JACC Focus Seminar. J Am Coll Cardiol. Mar 19 2019;73(10):1207-1228. PubMed PMID: 30871704. doi:10.1016/j.jacc.2018.11.059
- Cook GA, Jr., Sandroni P. Management of headache and chronic pain in POTS. Auton Neurosci. Dec 2018;215:37-45. PubMed PMID: 29929839. doi:10.1016/j.autneu.2018.06.004
- Mack KJ, Johnson JN, Rowe PC. Orthostatic intolerance and the headache patient. Semin Pediatr Neurol. Jun 2010;17(2):109-16. PubMed PMID: 20541103. doi:10.1016/j.spen.2010.04.006
- Heyer GL, Fedak EM, LeGros AL. Symptoms predictive of postural tachycardia syndrome (POTS) in the adolescent headache patient. Headache. Jun 2013;53(6):947-53. PubMed PMID: 23574111. doi:10.1111/head.12103
- Kapoor WN, Smith MA, Miller NL. Upright tilt testing in evaluating syncope: a comprehensive literature review. Am J Med. Jul 1994;97(1):78-88. PubMed PMID: 8030660. doi:10.1016/0002-9343(94)90051-5
- van Dijk N, Boer KR, Colman N, et al. High diagnostic yield and accuracy of history, physical examination, and ECG in patients with transient loss of consciousness in FAST: the Fainting Assessment study. J Cardiovasc Electrophysiol. Jan 2008;19(1):48-55. PubMed PMID: 17916139. doi:10.1111/j.1540-8167.2007.00984.x
- Benditt DG, Ferguson DW, Grubb BP, et al. Tilt table testing for assessing syncope. American College of Cardiology. J Am Coll Cardiol. Jul 1996;28(1):263-75. PubMed PMID: 8752825. doi:10.1016/0735-1097(96)00236-7
Declarations/Disclosures
Availability Of Data and Materials
Anonymized data will be shared, upon request, with any qualified investigator.
Authors’ Contributions
MS drafted the manuscript. MS, FF-T, LZ, NC designed and conceptualized the study and with MS, FF-T, RS, FJ, KM, KB, and MH played major roles in acquisition of data. MK and NT analyzed and interpreted the data. MS, FF-T, LZ, RS, FJ, NT, KM and NC all revised the manuscript for intellectual content.
Funding: This study was supported by a grant from the Bakken Heart-Brain Institute, Cleveland Clinic, Cleveland, Ohio.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
