Author: Audrey M Blazek * MD 1, Christopher P Rhyne MD 3, Ivan Da Silva MD, PhD 2, Bichun Ouyang PhD 2, Ellie Kothmann 3, Rima M Dafer MD, MPH 2
Author Affiliation:
1 Mayo Clinic, Department of Neurology, 200 First St. SW, Rochester, MN 55905
2 Rush University Medical Center, Department of Neurological Sciences, 1725 W Harrison St Suite 1106, Chicago, IL 60612
3 Diamond Headache Clinic, 1460 N Halsted St, Chicago, IL, 60642
Competing Interests: The author/s declare no competing interests.
Issue: 11.01
DOI: 10.30756/ahmj.2023.11.01
Received: Jun 15, 2023
Revised: Jul 26, 2023
Accepted: Aug 23, 2023
Published: Sept 5, 2023
Recommended Citation: Blazek AM, Rhyne CP, Silva ID, etal. Combined Prophylaxis of Chronic Migraine with OnabotulinumtoxinA and Anti-CGRP Antibodies. Ann Head Med. 2023;11:01. DOI: 10.30756/ahmj.2023.11.01
Objective: To evaluate the efficacy of adding large molecule anti-calcitonin gene-related peptide monoclonal antibodies (mAbs) to onabotulinumtoxinA (OBT-A) for chronic migraine prevention.
Background: Chronic migraine (CM) is a highly prevalent, debilitating disorder that leads to personal, social, and economic burdens. Both OBT-A and mAbs are proven safe and effective in chronic migraine prevention. The use of combination therapy has not been formally studied but may prove more effective than either monotherapy alone in select patients.
Methods: This is a retrospective chart review of patients with chronic migraine, treated with OBT-A, who received additional mAb preventative therapy. The primary endpoint was migraine headache days per month (MHD) after 3 months of combined therapy. Secondary endpoints included total headache days per month, headache intensity, disability level, and use of abortive medications.
Results: Of 1503 patients reviewed, 133 met inclusion criteria. At 3 months of combined therapy, mean reduction of MHD from baseline was 6.2 (95% CI 4.91 to 7.49, p < 0.0001). Nearly 2/3rd of patients (60.9%) experienced ≥ 50% MHD reduction. Fewer patients reported high disability level (14.6% [17/126], compared to 29.4%) and high headache intensity (13.0% [16/119], compared to 51.3%) at 3 months (all p < 0.0001). A reduction in abortive medication doses needed and discontinuation of concomitant oral preventative medications was also noted in 15.8% and 6.8% of patients, respectively.
Conclusions: Combined therapy with OBT-A and mAbs is well-tolerated, effective in further reducing migraine frequency, and may improve quality of life for patients with CM refractory to monotherapy.
Abbreviations
CM: chronic migraine
OBT-A: onabotulinumtoxinA
CGRP: calcitonin gene-related peptide
FDA: Food and Drug Administration
mAb: monoclonal antibody
EMR: electronic medical record
MHD: migraine headache days per month
THD: total headache days per month
CI: confidence interval
SD: standard deviation
MIDAS: migraine disability assessment scale
Introduction
Chronic migraine (CM) is a disabling medical condition defined as headache occurring ≥ 15 days per month for over 3 months with migraine features on ≥ 8 days per month 1. The worldwide prevalence of CM is estimated at 1.4-2.2% 2. Considering the high personal, social, and economic burden of CM, there is strong motivation for prophylactic treatment. Prevention requires a well-rounded treatment plan, and early focus on migraine prevention can reduce headache frequency, severity, disability, and abortive medication use. Migraine pathophysiology is complex, and a multimodal approach to treatment using layered preventative agents that target different pathways may prove beneficial to patients with CM 3, 4. This study aimed to examine the potential benefit of combined therapy of adding a large molecule anti-CGRP monoclonal antibody (mAb) to a pre-existing onabotulinumtoxinA (OBT-A) regimen in treatment refractory patients. We hypothesized that combined therapy results in fewer migraine headache days per month compared to monotherapy.
OBT-A was approved for CM prevention in 2010 and is safe, effective, and well-tolerated 5, 6. It is typically administered every 3 months across 31 injection sites in 7 muscles of the head and neck 2. In 2018, the US Food and Drug Administration (FDA) approved three mAbs for the prevention of CM: erenumab, galcanezumab, fremanezumab 7-9. A fourth mAb, eptinezumab, was approved in 2020 10. The use of mAbs has rapidly decreased the use of conventional, non-migraine-specific oral preventive agents including beta-blockers, calcium-channel blockers, tricyclic antidepressants, serotonin antagonists, serotonin norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, anticonvulsants, and antihypertensives 2. The mAbs are typically administered subcutaneously once per month, although fremanezumab can be administered quarterly, and eptinezumab is administered quarterly by intravenous route 10. These agents are usually well-tolerated, with a minimal side effect profile including pain or rash at the injection site, flu-like symptoms, and constipation 7-10.
The treatment of chronic migraine is challenging, especially for patients who are refractory to multiple preventative agents. There is evidence that patients with CM who have had a partial response to OBT-A but continue to experience frequent migraines may further benefit clinically from the addition of mAbs 11-13. This is thought to be due to their potentially additive, or even synergistic, mechanisms of action.
To date, there have been no randomized controlled trials to assess safety, efficacy, and cost-effectiveness of adding mAbs to OBT-A for chronic migraine prevention. In this study, we collected real-world data from the electronic medical records (EMR) of patients with CM treated at a single headache center. The potential benefit of combined therapy was based on data that was available in patients’ charts and commonly recognized as dependable and applicable for chronic migraine surveillance.
Methods
Study Design
This was a retrospective, longitudinal chart review of eligible patients treated at a comprehensive, multidisciplinary headache practice in Chicago, Illinois, USA between May 2018 and September 2021, in collaboration with Rush University Medical Center. A diagram of the study design can be found in Figure 1. Data were recorded at baseline, defined as just prior to the start of combined therapy, and after at least 2 consecutive courses, or 6-month duration, of OBT-A. Charts were reviewed up to several years prior to baseline to assess response to OBT-A as monotherapy and to determine eligibility, and up to 12 months after the start of combined therapy with OBT-A and mAb.
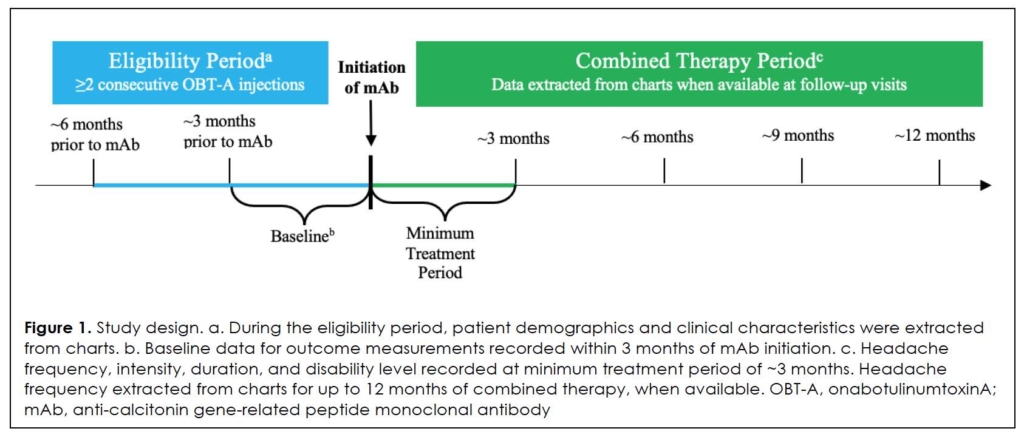
Setting And Population
We were provided de-identified data of patients with CM who were prescribed mAbs. We included patients over the age of 18 who were treated with ≥ 2 courses of OBT-A (6 months duration), based on the PREEMPT trials 5, 6. Patients were included only if they experienced at least 30% reduction in migraine headache days per month (MHD) on OBT-A alone and continued to have ≥ 3 MHD, resulting in the addition of a mAb 14. We excluded patients who (1) received OBT-A for any diagnosis other than CM, (2) received combined therapy for < 3 months, and (3) required adjustment of preventative medication or were started on new medications just prior to or during the first 3 months of combined therapy. The target sample size was approximately 250 patients in order to assess effectiveness of combined treatment.
Data Collection
The institutional review board at Rush University Medical Center approved the study protocol and determined that requirements were met for waiver of consent. Patient demographics, including year of birth, sex, and race were obtained through chart review of the EMR.
The primary study endpoint was defined as percent reduction in MHD from baseline after 3 months of combined therapy. Secondary endpoints included percent reduction in total headache days per month (THD) from baseline after 3, 6, 9, and 12 months of combined therapy when documented, and changes in headache intensity and duration, disability level, and number of abortive medications being used after 3 months. Final analyses were performed using data that were available for the majority of patients. MHD after more than 3 months of combined therapy were not always collected due to the exploratory nature of the retrospective study and variations in data reporting.
Other clinical data collected included number of abortive medication doses used, concomitant medications, start date of OBT-A and mAb, type of mAb, dose, and reason for discontinuation, when available. Changes in mAb dose, brand, and side effects were noted if documented.
Endpoint Assessments
MHD and THD were self-reported by patients during clinic visits. Methods of reporting varied by patient and clinician. There was no standardized note template or questionnaire used to collect data due to the retrospective nature of the study. Due to the self-reporting nature of this study, when unable to differentiate between non-migraine headache days and migraine headache days during data collection, all headaches were presumed migraines, and MHD equaled THD.
Headache intensity was documented based on visual analog scale, with 10 being most severe, or as low, moderate, or high intensity. The quantitative value was converted to qualitative: 0-3 (low), 4-7 (moderate), and 8-10 (high).
Headache duration was documented in minutes, hours, or days, and subsequently converted to hours. When a patient described a headache that was constant or lasting all day, the duration was recorded as 24 hours.
Validated migraine disability scales such as the migraine disability assessment scale (MIDAS) was not used at this headache practice 15. Disability level was documented in several ways: days per month the patient was able to function at < 50% of normal capacity, days per month the patient was unable to attend work, school, or other commitments, or documented as low, moderate, or high disability. When documented as days per month, the disability level was converted to a qualitative value: 0-1 days (low), 2-6 days (moderate), and ≥ 7 days (high). These values were based on the MIDAS, which indicates severe disability as 21 days or more in 3 months (or 7 days or more in 1 month) on which the patient was unable to fully function or take part in daily, leisure, work, or school related activities.
Data Analysis
Descriptive statistics were used to summarize data. Mean reduction in MHD from baseline with corresponding 95% confidence intervals (CIs) were calculated. Proportions of patients who experienced ≥ 50%, ≥ 75%, and 100% reduction in MHD were calculated. THD up to 12 months of combined therapy were analyzed using ANOVA. Decrease in disability level and headache intensity were analyzed using the McNemar-Bowker test.
Due to the exploratory nature of this longitudinal, real-world study and the collection of data originally documented for patient care rather than research, there were missing values. This may be due to data being not relevant to the patient or unavailable in patients’ charts. Patients with missing data after 3 months of combined therapy were excluded from analysis at the 6, 9, and 12-month timepoints.
Results
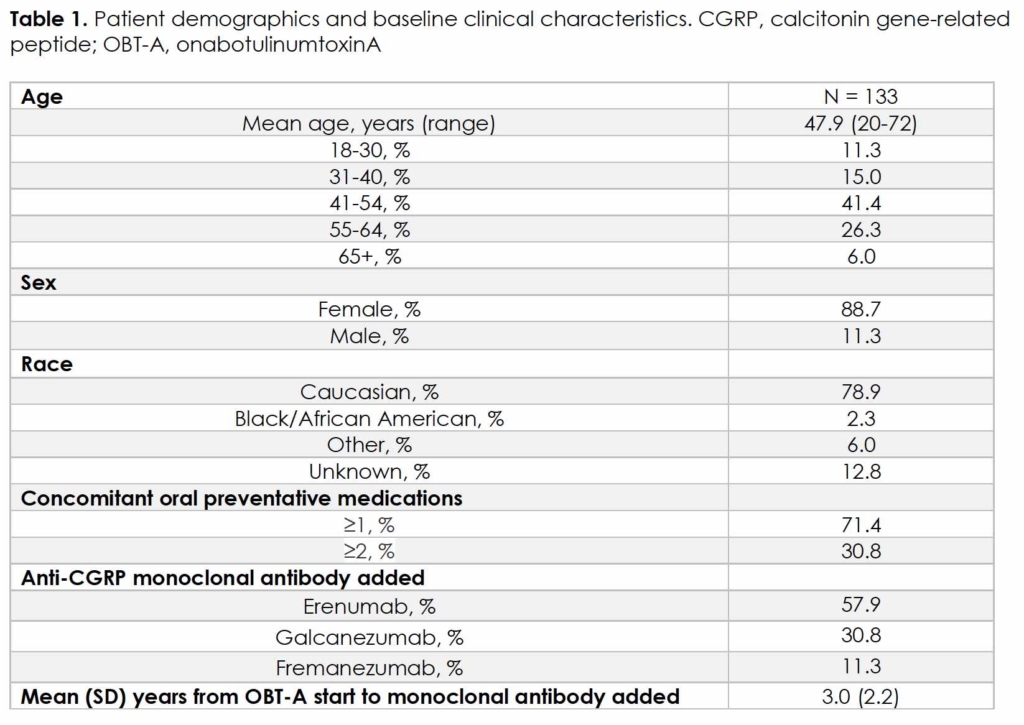
Of the 1503 patients prescribed mAbs, 133 met this study’s inclusion criteria, as shown in Figure 2. Demographics and clinical characteristics are summarized in Table 1. Mean age was 47.9 years (range, 20-72), with the majority being female (88.7%) and Caucasian (78.9%). Mean (SD) time from OBT-A initiation to addition of mAb was 5.4 (2.6) years. Out of 133 patients, 131 received OBT-A for more than 2 cycles (6 months) prior to mAb addition, and 119 received OBT-A for at least 2 years prior to mAb addition. A total of 71.4% and 30.8% of patients were on concomitant ≥ 1 or ≥ 2 oral preventative medications, respectively, at the time of mAb addition, including 49.6% Level A agents per AHS/AAN guidelines 16. Patients requiring increased dose or additions of new oral preventative medications for a diagnosis other than CM in the first 3 months of combined therapy were excluded from this analysis.
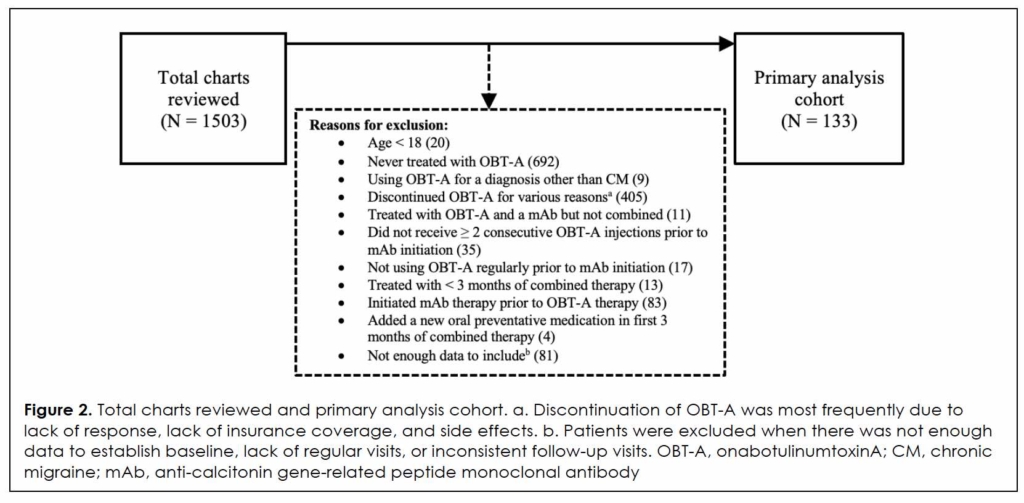
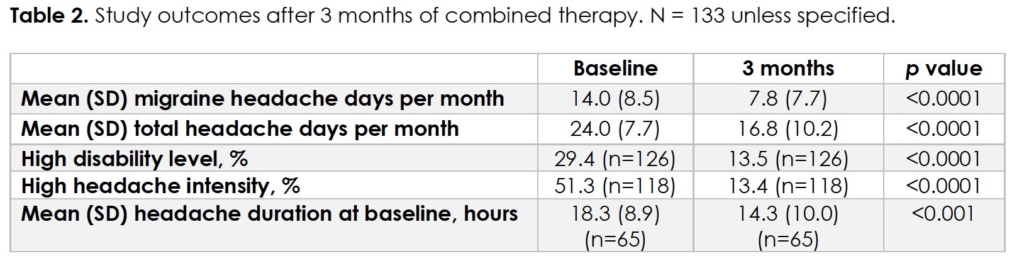
At baseline, mean (SD) MHD and THD were 14.0 (8.5) and 24.0 (7.7), respectively (Table 2). High disability level was reported by 29.4% (37/126) of patients, and 51.7% reported high headache intensity (61/118). Mean headache duration was 18.3 hours (n = 65).
At 3 months of combined therapy, mean (SD) MHD and THD were 7.8 (7.7) and 16.8 (10.2), respectively (p < 0.0001). There was a mean reduction of 6.2 MHD (95% CI 4.91 to 7.49) and 7.2 THD (95% CI 5.87 to 8.53) from baseline. Over half (60.9%) of patients experienced ≥ 50% reduction in MHD, 24.8% experienced ≥ 75% reduction, and 6.8% experienced 100% reduction.
There was a consistent effect of combined therapy at 3, 6, and 12 months compared to baseline. (all p < 0.0001), resulting in a least square mean (standard error) THD of 24.0 (0.9) at baseline, 16.8 (0.9) at 3 month, 17.5 (0.9) at 6 months, and 17.0 (0.9) at 12 months.
There were significant changes in disability level at 3 months of combined therapy compared to baseline (p < 0.0001), as seen in Figure 3. Of the patients with low disability at baseline, 97.0% (34/35) remained low. Of the patients with moderate disability at baseline, 38.9% (21/54) decreased to low. Of the patients with high disability at baseline, 32.4% (12/37) decreased to moderate, and 24.3% (9/37) decreased to low.
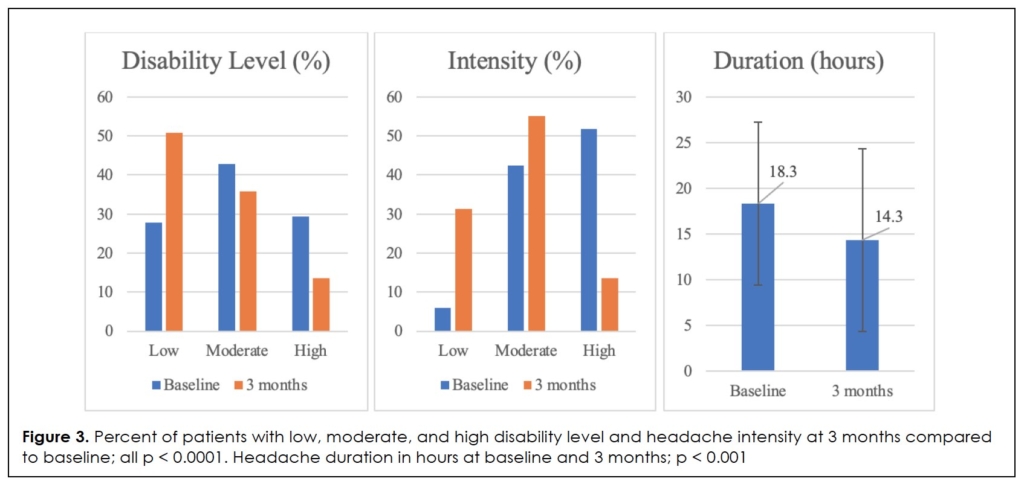
There was a similarly significant reduction in headache intensity (p < 0.0001), with 100% (7/7) of patients reporting low headache intensity at baseline remaining low at 3 months. Of the patients reporting moderate intensity at baseline, 48.0% (24/50) decreased to low, and of those reporting high intensity at baseline, 65.6% (40/61) decreased to moderate, and 9.8% (6/61) decreased to low.
There was a significant reduction in mean headache duration to 14.3 hours (p < 0.001). A reduction in total doses of abortive medication was noted in 21 patients (15.8%). Nine patients (6.8%) discontinued at least one concomitant oral preventative medication due to reduction in headache frequency.
Combined therapy was discontinued in 26.3% (35/133) of patients by 12 months. Commonly reported reasons for treatment discontinuation included insufficient response (12/35), lack of insurance coverage (5/35), and adverse effects (4/35). The most commonly-reported adverse effects were constipation and mild injection site reaction. No serious adverse events were documented.
Discussion
Treatment of chronic migraine is a complex and multifaceted task for clinicians. The impact of migraines on an individual patient’s life must be taken into account, as disease burden is widely variable. Many patients experience significant social and emotional burdens due to high disability level and psychologic co-morbidities. Financial concerns at a personal level and across the healthcare system are also substantial due to the costs of emergency room visits, hospitalizations, and lack of insurance coverage for multiple therapies at one time. These are barriers that can lead to ineffective treatment overall and chronification of migraine. Treatment regimens should be personalized in order to meet a patient’s goals and circumstances.
To optimize treatment of chronic migraine, certain patients may require more than a monotherapy approach, such as combined therapy with OBT-A and mAbs. Using a layered approach involving older and newer therapies, and non-traditional options, allows for the targeting of multiple pathways involved in migraine pathophysiology. It can also be tailored to an individual’s goals, as explained in a recently-published statement by the American Headache Society 17. OBT-A and mAbs have been separately approved for chronic migraine prevention, and many clinicians add mAbs to pre-existing OBT-A regimens in their routine practice. There is limited data and no randomized controlled trials focusing on the safety and effectiveness of combined therapy. Recent studies have shown preliminary evidence that such combined therapy may reduce migraine frequency and improve quality of life in patients with CM due to their unique additive or synergistic mechanisms of action.
In this real-world, retrospective study of 133 patients with CM, combined therapy of OBT-A and mAb was effective in reducing the number of migraine and total headache days per month, disability level, and headache intensity. Combined therapy was generally well-tolerated. Our data are similar to those reported in other recently-published studies by Mechtler et al, and Blumenfeld et al 16, 18.
Findings of the COMPEL trial were taken into consideration 19. Despite inclusion criteria based on PREEMPT trials of a 2 cycle (6 month) trial of OBT-A prior to mAb addition, almost 90% of patients in this study received at least 2 years of OBT-A therapy prior to starting a mAb. The COMPEL trial showed that patients continued to experience reduction in migraine headache days per month up to 108 weeks after the first injection. That timeframe is consistent with this study, as the majority of patients did not initiate mAb therapy due to a plateau in MHD until at least 2 years of OBT-A injections.
The subset of patients in this study appeared to experience a greater reduction in MHD compared to those in other recent publications. In this study, the primary endpoint was at 3 months of combined therapy. It is possible that if MHD had been consistently available for up to 12 months of combined therapy, a mean reduction in MHD would have been smaller and more similar to that seen in other studies.
We used migraine frequency, intensity, duration, disability level, and response to abortive medications as assessments, when available in patients’ charts. This differs from many recent studies, which commonly use the MIDAS to measure headache disability. While these values were not available during chart review, reporting and analyzing the measurements we did record are similar representations of response to combined therapy. Migraine frequency is an important measure of response to treatment, but quality of life is also impacted by headache intensity, duration, days of work or school missed, engagement in daily activities, and response to abortive medications. It is challenging to determine what constitutes improvement in quality of life for an individual using the subjective nature of data that was documented without the MIDAS available. The lack of uniform disability measurements could be considered a limitation of this study. Of note, many patients who did not have seemingly significant reduction in migraine frequency continued on combined therapy for more than 3 months, suggesting good tolerability of mAbs. Patients reported improved focus and engagement, less severe headaches, increased responsiveness to abortive medications, and headaches that were overall more manageable than prior to combined therapy.
There are several other limitations to this study. This is a retrospective chart review analysis using data originally recorded for patient care rather than for research purposes. Resultingly, there were missing values throughout the study period that prevented us from analyzing other variables that may prove beneficial in determining clinical benefit of combined therapy. The frequency of migraines and total headache days per month were not routinely recorded for every patient beyond the 3 months of combined therapy, nor were intensity, duration, disability level, and number and dose of abortive medications used. This introduces bias, despite medication discontinuation and loss to follow-up being common in clinical practice. Ideally, a prospective study would be performed in which patients used a diary to record headache characteristics and medication use. Patients would be monitored, and data recorded at specific timepoints. This would allow for more consistent and accurate data that could be used for research purposes. Additionally, the dose of OBT-A administered and the timing of OBT-A administration with mAb injections was not recorded. Both of these variabilities may change the clinical effect of combined therapy, as wearing-off is a well-known effect of both OBT-A and Abs (except galcanezumab 20). Administering both therapies at the same point in time may produce a smaller clinical benefit than if patients were to alternate therapies to reduce the wearing-off period. The start date of mAb was not always recorded to an exact date, as patients often self-injected at home. Therefore, the 3, 6, 9, and 12-month timepoints are approximate. Because the study period started the first year mAbs were approved by the FDA, there is a greater proportion of patients who used erenumab (57.9%) compared to the other two brands, and it was typically administered at 70 mg/mL despite a dose of 140 mg/mL being the more commonly prescribed dose now that more studies have emerged showing greater clinical benefit at a higher dose.
Conclusion
Combined therapy with onabotulinumtoxinA and anti-CGRP monoclonal antibodies in patients with chronic migraine refractory to monotherapy is well-tolerated and could offer additional benefit with further reduction in migraine frequency, headache intensity, disability level, and overall quality of life. Further data from randomized clinical trials may shed light on additional benefit and assess long-term safety and efficacy of prophylactic combined therapy.
Tables
References
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. Jan 2018;38(1):1-211. PubMed PMID: 29368949. doi:10.1177/0333102417738202
- Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. Aug 30 2019;20(1):92. PubMed PMID: 31470791; PubMed Central PMCID: PMCPMC6734211. doi:10.1186/s10194-019-1038-4
- Salem-Abdou H NP, Puymirat J. Erenumab and Onabotulinumtoxin A Show Additive Effect in Refractory Chronic Migraine. Pract Pain Manag. Mar/Apr 2020;20(2):47-49.
- Talbot J, Stuckey R, Crawford L, Weatherby S, Mullin S. Improvements in pain, medication use and quality of life in onabotulinumtoxinA-resistant chronic migraine patients following erenumab treatment – real world outcomes. J Headache Pain. Jan 9 2021;22(1):5. PubMed PMID: 33421995; PubMed Central PMCID: PMCPMC7797151. doi:10.1186/s10194-020-01214-2
- Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. Jul 2010;30(7):793-803. PubMed PMID: 20647170. doi:10.1177/0333102410364676
- Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. Jul 2010;30(7):804-14. PubMed PMID: 20647171. doi:10.1177/0333102410364677
- Goadsby PJ, Reuter U, Hallstrom Y, et al. A Controlled Trial of Erenumab for Episodic Migraine. N Engl J Med. Nov 30 2017;377(22):2123-2132. PubMed PMID: 29171821. doi:10.1056/NEJMoa1705848
- Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology. Dec 11 2018;91(24):e2211-e2221. PubMed PMID: 30446596; PubMed Central PMCID: PMCPMC6329331. doi:10.1212/WNL.0000000000006640
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the Preventive Treatment of Chronic Migraine. N Engl J Med. Nov 30 2017;377(22):2113-2122. PubMed PMID: 29171818. doi:10.1056/NEJMoa1709038
- Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. Mar 31 2020;94(13):e1365-e1377. PubMed PMID: 32209650; PubMed Central PMCID: PMCPMC7274916. doi:10.1212/WNL.0000000000009169
- Shivangi Singh HS, Raghav Govindarajan. Galcanezumab in the Prevention of Chronic Migraine in Patients on Botulinum Toxin Therapy (5257). Neurology. Apr 2020 2020;94 (15 Supplement):5257.
- Ailani J, Pearlman E, Zhang Q, Nagy AJ, Schuh K, Aurora SK. Positive response to galcanezumab following treatment failure to onabotulinumtoxinA in patients with migraine: post hoc analyses of three randomized double-blind studies. Eur J Neurol. Mar 2020;27(3):542-549. PubMed PMID: 31595600; PubMed Central PMCID: PMCPMC7028018. doi:10.1111/ene.14102
- Pellesi L, Do TP, Ashina H, Ashina M, Burstein R. Dual Therapy With Anti-CGRP Monoclonal Antibodies and Botulinum Toxin for Migraine Prevention: Is There a Rationale? Headache. Jun 2020;60(6):1056-1065. PubMed PMID: 32437038. doi:10.1111/head.13843
- Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. May 2008;28(5):484-95. PubMed PMID: 18294250. doi:10.1111/j.1468-2982.2008.01555.x
- Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20-8. PubMed PMID: 11294956. doi:10.1212/wnl.56.suppl_1.s20
- Mechtler L, Saikali N, McVige J, Hughes O, Traut A, Adams AM. Real-World Evidence for the Safety and Efficacy of CGRP Monoclonal Antibody Therapy Added to OnabotulinumtoxinA Treatment for Migraine Prevention in Adult Patients With Chronic Migraine. Front Neurol. 2021;12:788159. PubMed PMID: 35069416; PubMed Central PMCID: PMCPMC8770868. doi:10.3389/fneur.2021.788159
- Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. Apr 24 2012;78(17):1337-45. PubMed PMID: 22529202; PubMed Central PMCID: PMCPMC3335452. doi:10.1212/WNL.0b013e3182535d20
- Blumenfeld AM, Frishberg BM, Schim JD, et al. Real-World Evidence for Control of Chronic Migraine Patients Receiving CGRP Monoclonal Antibody Therapy Added to OnabotulinumtoxinA: A Retrospective Chart Review. Pain Ther. Dec 2021;10(2):809-826. PubMed PMID: 33880725; PubMed Central PMCID: PMCPMC8586140. doi:10.1007/s40122-021-00264-x
- Blumenfeld AM, Stark RJ, Freeman MC, Orejudos A, Manack Adams A. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. Feb 5 2018;19(1):13. PubMed PMID: 29404713; PubMed Central PMCID: PMCPMC5799088. doi:10.1186/s10194-018-0840-8
- Ailani J, Kuruppu DK, Rettiganti M, et al. Does “wearing off” of efficacy occur in galcanezumab-treated patients at the end of the monthly treatment cycle? Post hoc analyses of four phase III randomized trials. Headache. Feb 2022;62(2):198-207. PubMed PMID: 35076090; PubMed Central PMCID: PMCPMC9306502. doi:10.1111/head.14257
Acknowledgements
Thank you to Diamond Headache Clinic for use of their electronic medical record in order to study their patient population for the purpose of this research.
Declarations/Disclosures
Consent/Permission/Ethics Approval: Not applicable.
Funding/Conflicts of interest: In compliance with the ICMJE uniform disclosure form, author declares the following:
Payment/services info: Author has declared that no financial support was received from any organization for the submitted work.
Financial relationships: Author have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: Author have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
