Author: Dev G. Mehta DO* 1,2, Robert Gurianov MD 3, Victor A. Gombolevskiy MD, PhD, MPH 4, Sergey Morozov MD, PhD 4, Carrie E. Robertson MD 2, Narayan R. Kissoon MD 2,5, Nirusha Lachman PhD 6, Sebastian Cotofana MD, PhD, PhD 6
Author Affiliation:
1 Department of Neurology, University of Washington, Seattle, WA, USA
2 Department of Neurology, Mayo Clinic, Rochester, MN, USA
3 Department of Oncology and Radiotherapy and Department of Plastic Surgery, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
4 Department of Research and Practical Center of Diagnostics and Telemedicine Technologies of the Moscow Health Care Department, Moscow, Russia
5 Department of Anesthesiology, Mayo Clinic, Rochester, MN, USA
6 Department of Clinical Anatomy and Department of Plastic Surgery, Mayo Clinic College of Medicine and Science, Rochester, MN, USA
Competing Interests: The author/s declare no competing interests.
Issue: 04.02
DOI: 10.30756/ahmj.2020.04.02
Received: Dec 29, 2020
Revised: Jan 10, 2021
Accepted: Jan 12, 2021
Published: Jan 22, 2021
Recommended Citation: Mehta DG, Gurianov R, Gombolevskiy VA, et al. A Computerized Tomographic and Cadaveric Study of Sphenopalatine Ganglion Block Via the Pterygomaxillary Fissure: Anatomical Considerations of the Suprazygomatic Approach. Ann Head Med. 2020;04:02. DOI: 10.30756/ahmj.2020.04.02
Background: The sphenopalatine ganglion (SPG) is a parasympathetic ganglion that’s implicated in multiple primary headache disorders. Current techniques are inconsistent or require imaging. A suprazygomatic approach is thought to be safe and effective. The main objectives are to determine an accurate depth and needle angulation to perform SPG blocks safely and effectively.
Methods: Cranial computerized tomography was obtained from 40 Caucasians (20 male, 20 female). For each patient, a line is drawn from the frontozygomatic angle to the pterygomaxillary fissure (PMF) to represent length. Intersection of the line in both transverse and coronal Frankfurt planes create inferior and posterior angulations, respectively.
A cadaver dissection is performed to validate the needle placement utilizing these measurements. Contrast fluoroscopy is utilized to verify needle placement in PMF.
Results: The mean length was 24.9 +/- 2.8mm for males and 23.8 +/- 0.5mm for females. The difference was statistically significant (p = .019). In patients younger than 40 years, the mean length was 24.5 +/- 2.8mm. In patients greater than 40 years, the mean length was 26.1 +/- 3.1mm. The difference was statistically significant (p = .018). Both inferior and posterior angles were not statistically different between sex or age. Post-contrast imaging confirmed presence of dye in the PMF.
Conclusion: While there is a difference in depth between sex and age groups, it’s likely not clinically significant. A depth of 25mm angulated at 6 degrees inferior and 27 degrees posterior is likely generalizable. Bony landmark-based, suprazygomatic SPG blocks via the PMF are probably feasible, safe with minimal risk and may be an option in special patient populations and/or circumstances.
Introduction
The sphenopalatine ganglion (SPG) is the largest, extracranial, predominately parasympathetic ganglion 1. It is primarily innervated by the greater petrosal branch of the facial nerve, a parasympathetic branch, which originates in the superior salivary nucleus in the dorsal pons. Post-ganglionic fibers travel with deep branches of the trigeminal nerve to the nose, soft palate, tonsils, uvula, and lacrimal gland. The SPG is located within the pterygopalatine fossa (PPF), which is an inverted pyramid- shaped depression deep to the infratemporal fossa, and accessible through the pterygomaxillary fissure (PMF) 2. It is located between the pterygoid process and the maxillary tuberosity close to the apex of the orbit.
Interventions targeting the SPG, including anesthetic blocks, have shown to provide benefits in both cluster headache and migraine 3, 4, 5. There are a number of approaches to performing the blocks with the most common method being a transnasal approach using pledgets, cue tips, and intranasal catheters, but these tend to have mixed responses likely related to anatomical variances in mucosal thickness in patients 6, 7. The most consistent and effective treatment response typically occurs with a lateral infrazygomatic percutaneous approach with fluoroscopic or CT guidance 8 . Imaging is used due to the risk of maxillary artery injury. Penetration of the base of the skull as well as the orbit has the potential to occur 9 .
Recent studies have shown that SPG blocks via a suprazygomatic approach can be safe and effective with some of these techniques better described as a pterygopalatine fossa field block 3, 8, 9, 10. However, the standardization of this technique is, to date, less well defined.
The main objective of this study is to identify a reproducible, safe and reliable access pathway via a suprazygomatic approach to target the SPG located in the PPF.
Methods
Study Sample
The study relied on the retrospective analysis of previously obtained cranial CT images from 40 Caucasians of Russian origin; of which there were 10 males and 10 females below the age of 40 and 10 males and 10 females above the age of 40. The mean age for all patients was 49.7 +/- 20.2 years [range: 22.0-80.0]. The mean weight for all patients was 75.6 +/- 14.8 kilograms [range: 54.0-112.0]. The mean height for all patients was 171.5 +/- 9.9 centimeters [range: 150-194]. The CT imaging results were verified with cadaveric dissections and fluoroscopic imaging conducted in one male body donor.
Computed Tomography Study Component
Cranial CT images were acquired from the Research and Practical Center of Medical Radiology of the Department of Health Care image database. Scans were obtained during previous routine cranial CT examinations. Scans showing cranial fractures or irregularities of cranial bones were not included in this analysis. Prior to entering the image database, each patient provided informed consent that they would allow researchers to use their CT scans and demographic data for scientific purposes in the future. The ethics committee of the Department of Health, Moscow, Russia approved the use of these scans for the purpose of this study (retrospective analysis of CT scans; protocol number 5).
CT images were generated by a Toshiba Aquilion 64 scanner (Toshiba Medical Systems Corporation, Otawara, Tochigi, Japan) utilizing the following parameters: 220-mm field of view, 0.5-mm slice thickness, automatically adapt the tube current (40-500 mA) with SureExp3D (SD is 10 for 5.0mm thickness), and 120-kV voltage. CT scans were imported into Syngo.via VB20 (SIEMENS Healtheneers, Germany), AGFA Enterprise (Agfa Healthcare), Mimics Innovation Suite software (Materialise NV, Leuven, Belgium), and OsiriX 8.0 (Pixmeo, Swiss) and the temporal region was bilaterally analyzed.
First, the injection site and target site were identified: the frontozygomatic angle and the PMF, respectively. To measure the length, a straight line was drawn from the ipsilateral frontozygomatic angle to the ipsilateral PMF. (Figure 1) This was done bilaterally to obtain the distance in mm on each side.
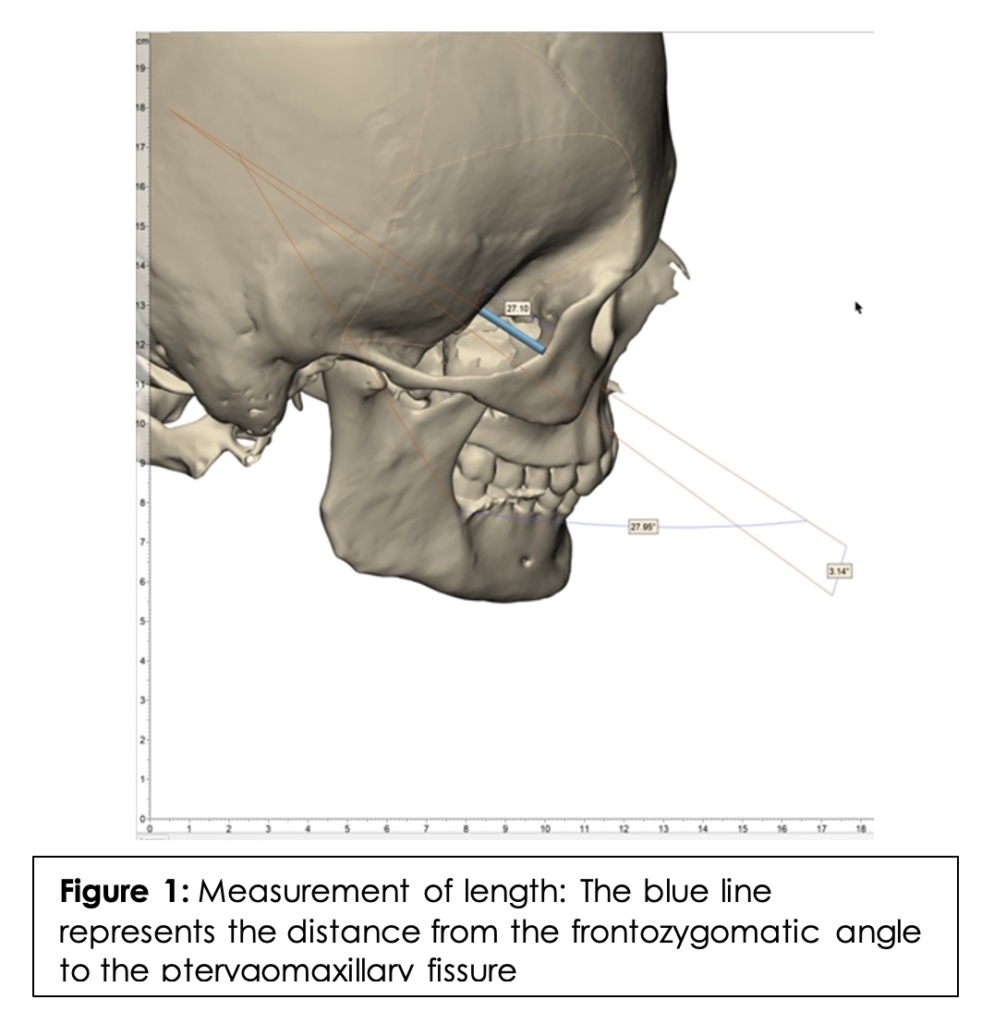
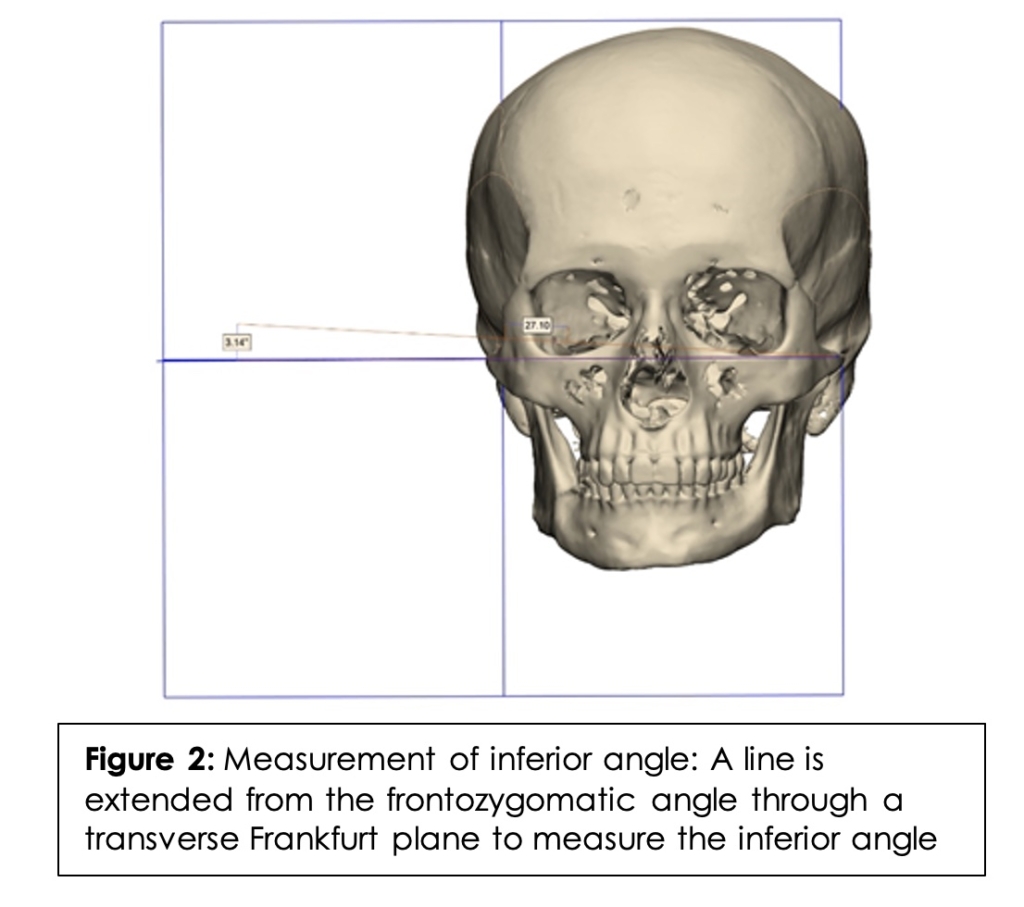
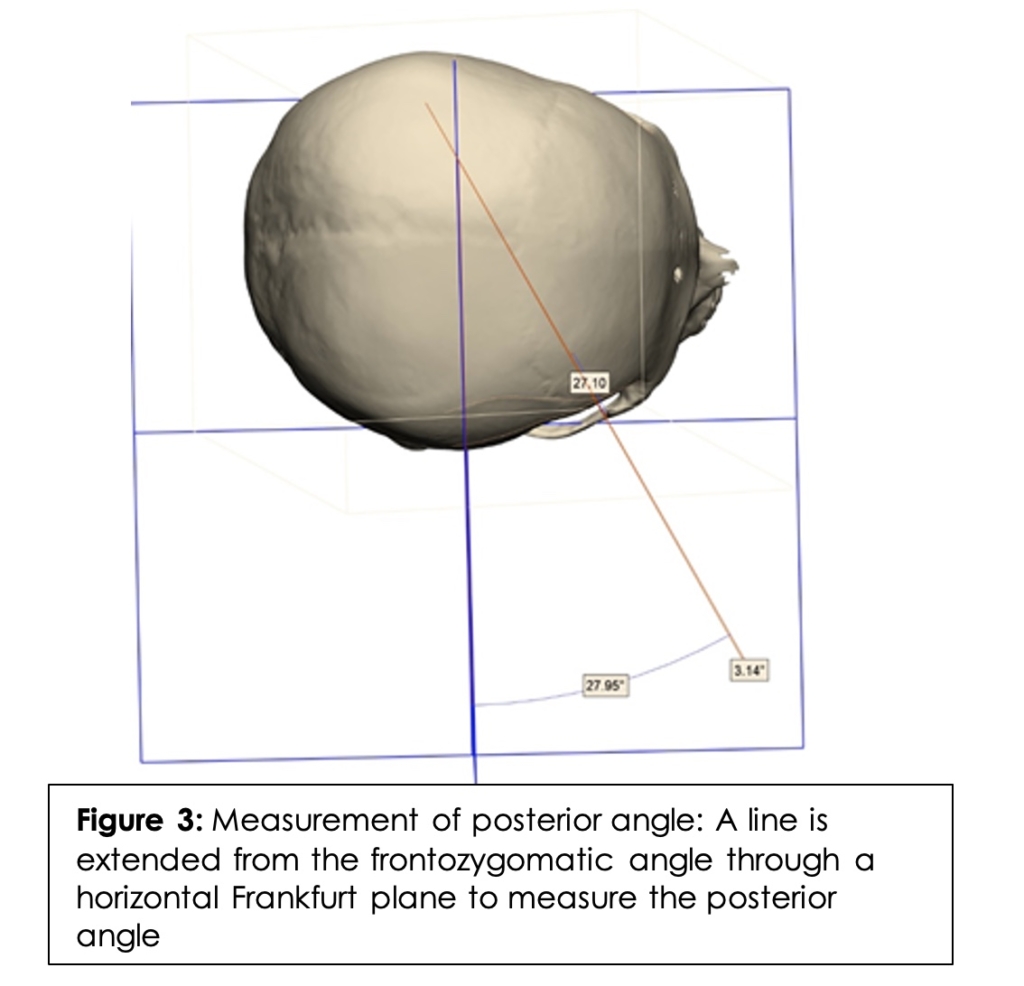
To measure the inferior angle, a straight line was drawn from the frontozygomatic angle to the PMF extending in each direction. (Figure 2) A transverse Frankfurt plane (the anatomical position of the human skull, based on a plane passing through the inferior margin of the orbit and the upper margin of each ear canal or external auditory meatus) was then added. The angle at which the line intersected the Frankfurt plane was considered the inferior angle. In order to measure the posterior angle, a straight line was drawn from the frontozygomatic angle to the PMF extending in each direction. A coronal Frankfurt plane was then added. (Figure 3) The angle at which the line intersected the coronal Frankfurt plane was considered the posterior angle.
Using the current sample population, the mean angle in all three planes and length was calculated along with standard deviation and range.
Cadaveric Study Component
The cadaveric study component was approved by the Mayo Clinic Biospecimens Committee (19-009344). Infratemporal fossa dissection and injection simulation were performed in a non-embalmed latex-injected male body donor.
Dissection Procedure
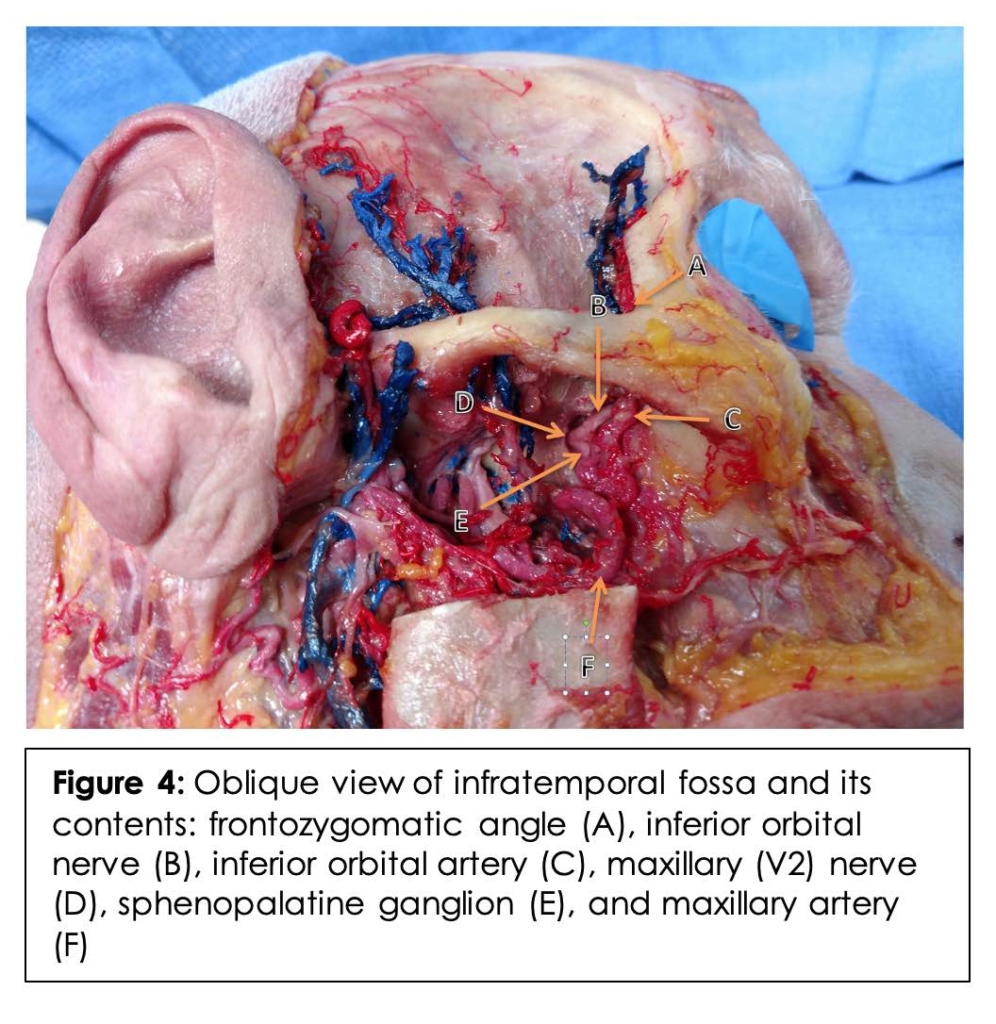
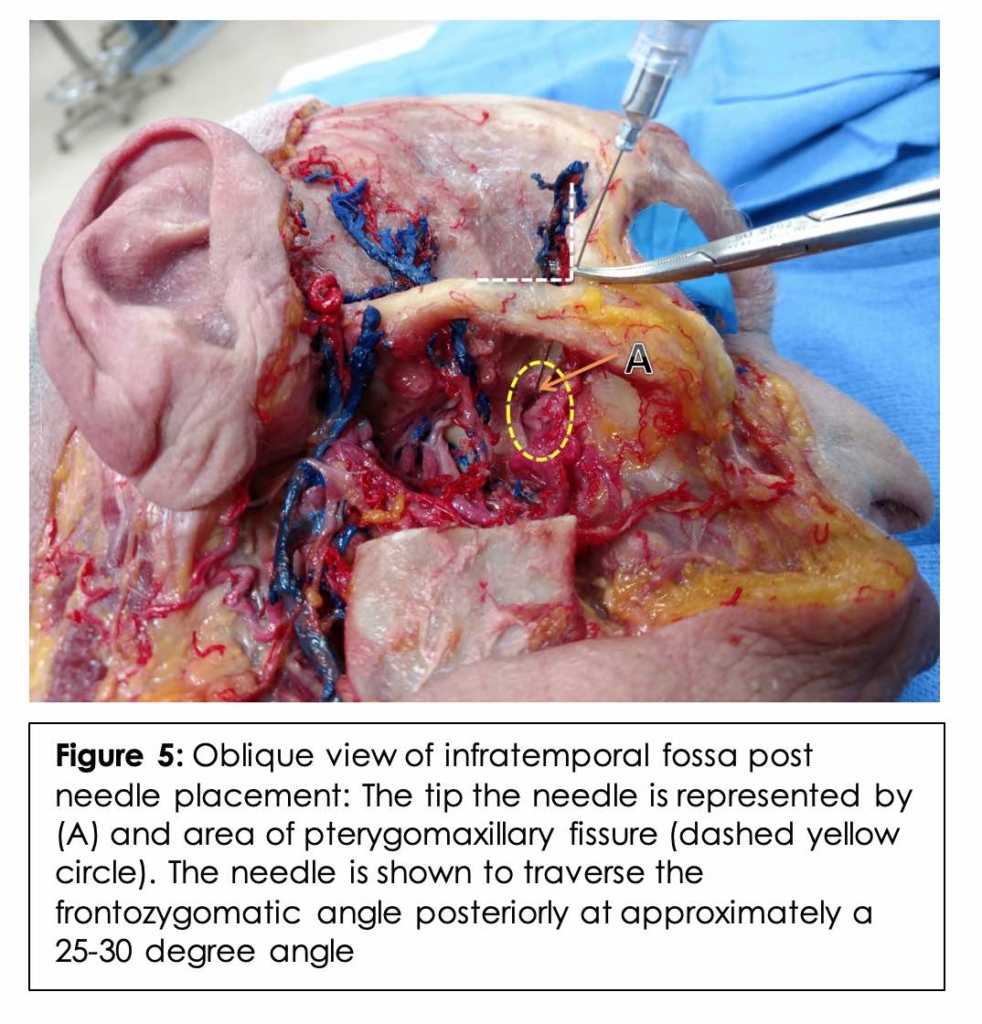
On the right side of the cadaver, dissection of the infratemporal fossa was performed with exposure of the maxillary nerve and artery along with their respective branches. Branches of the maxillary artery (sphenopalatine and infraorbital), branches of the maxillary nerve (infraorbital) and the SPG were carefully identified. (Figure 4) A simulated injection was then performed utilizing the above approximated mean angle and length. (Figure 5, 6A) The tip of the needle is seen lateral to the PMF in Figure 5.
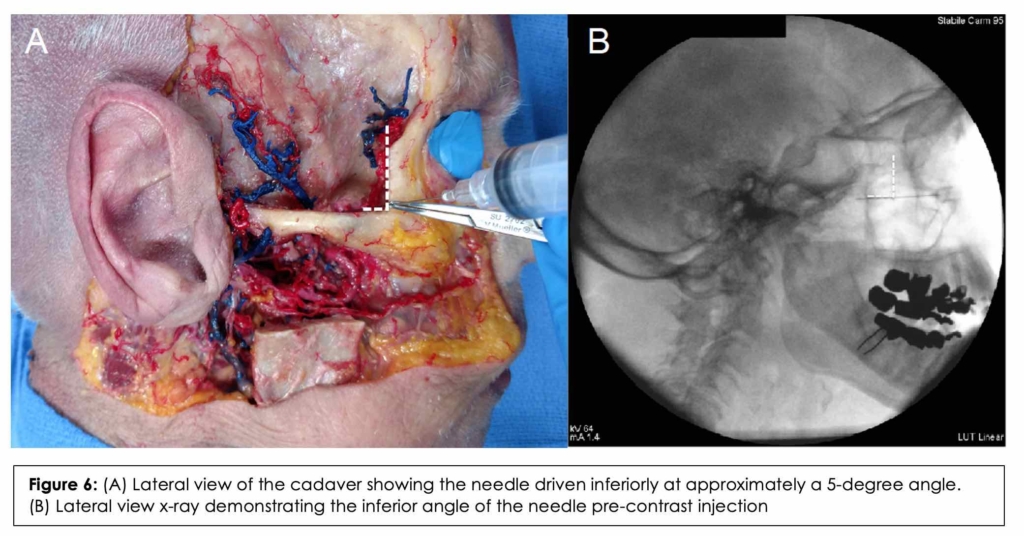
On the left side, the suprazygomatic approach was simulated under fluoroscopic imaging based on the results obtained from the CT imaging study component to validate the approach. The head of the body donor was fixed in a lateral decubitus position, and an injection was performed utilizing the previously calculated mean angles and length. A lateral cranial x-ray was performed to ensure correct needle placement. The cadaver was then injected with radiopaque contrast. A lateral cranial x-ray was obtained to ensure adequate spread into the PMF. A C-arm Siemens Arcadis x-ray machine was used to generate these images (Siemens AG, Munich, Germany) using the following parameters: 1.4 mA-tube current and 64-kV voltage.
Statistical Analysis
Direct comparison of groups was performed using a two-sample t-test. Descriptive statistical analysis was performed using SPSS 25.0 (IBM Corp., Armonk, NY).
Results
In both males and females, there was no statistical difference in length between sides (p = .385 and p= .638, respectively). Therefore, all other calculations for length were performed independently of side. The mean length from the frontozygomatic angle to the PMF (referred to as mean length) for males was 24.9 +/- 2.8 mm [range: 19-32] and for females it was 23.8 +/- 0.5 mm [range: 18.5-28.8]. There was a statistically significant difference noted between males and females (p = .019). In patients younger than 40 years of age, the mean length was 24.5 +/- 2.8 mm. In patients greater than 40 years of age, the mean length was 26.1 +/- 3.1 mm. There was a statistically significant difference between age groups (p = .018). The average body mass index (BMI) was 25.6 +/- 4.6 [range: 17.9-34.2]. There was no statistically significant difference in length, in either men or women, when comparing patients with a BMI below 25 and above 25 (p = .28, p = .112, respectively).
When comparing angulations from the right and left side in males, there was no statistically significant difference in either the inferior or posterior angles (p = .281 and p = .353, respectively). When comparing angulations from the right and left side in females, there was no statistically significant difference in either the inferior or posterior angles (p = .619 and p = .726, respectively). Therefore, all other calculations for angulations were performed independently of side. With the specimen in a lateral decubitus position with the nose being parallel to the floor, the mean angle of trajectory to most accurately approximate from the frontozygomatic angle to the PMF in males was 6.3 +/- 7.4 degrees [range: 0-48] inferior and 27.0 +/- 8.9 degrees [range: 8-48] posterior. The mean angle of trajectory in females was 6.0 +/- 5.0 degrees [range: 0-30] inferior and 28.4 +/- 10.6 degrees [range: 3-55] posterior. There was no statistical significance in either the inferior angle or posterior angle when comparing genders (p =.791 and p = .518, respectively). In patients younger than 40 years of age, the mean inferior angle was 5.8 +/- 6.6 degrees [range: 0-39] and the mean posterior angle was 27.6 +/- 10.0 degrees [range: 8-56]. In patients greater than 40 years of age, the mean inferior angle was 6.5 +/- 6.0 degrees [range: 0-30] and the mean posterior angle was 27.8 +/- 9.6 degrees [3-48]. There was no statistical significance in either the inferior or posterior angles when comparing age groups (p = 633, p = .919 respectively). To simulate the injection in our male cadaveric specimen, a 25-gauge 2-inch needle was clamped with a bent nose plier at 25mm. (Figure 7)
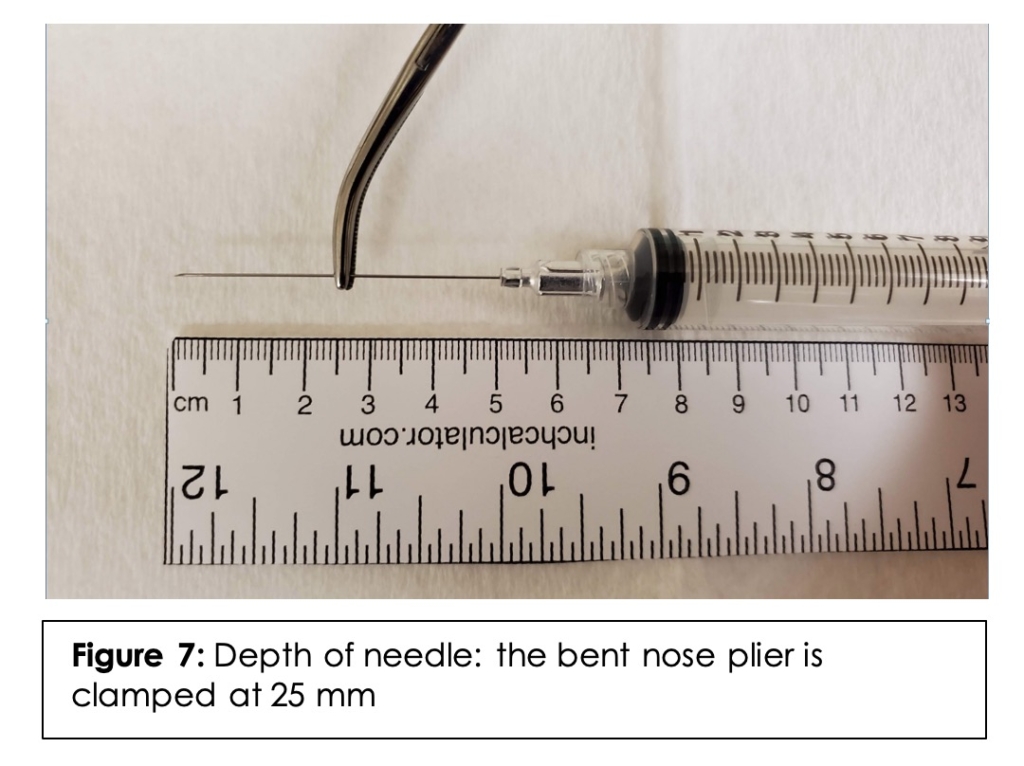
This depth was chosen as it is approximately the average depth (24.9mm) seen in our male specimens. Similarly, an approximated angle of 6 degrees inferior and 27 degrees posterior was used to reflect the averages noted above.
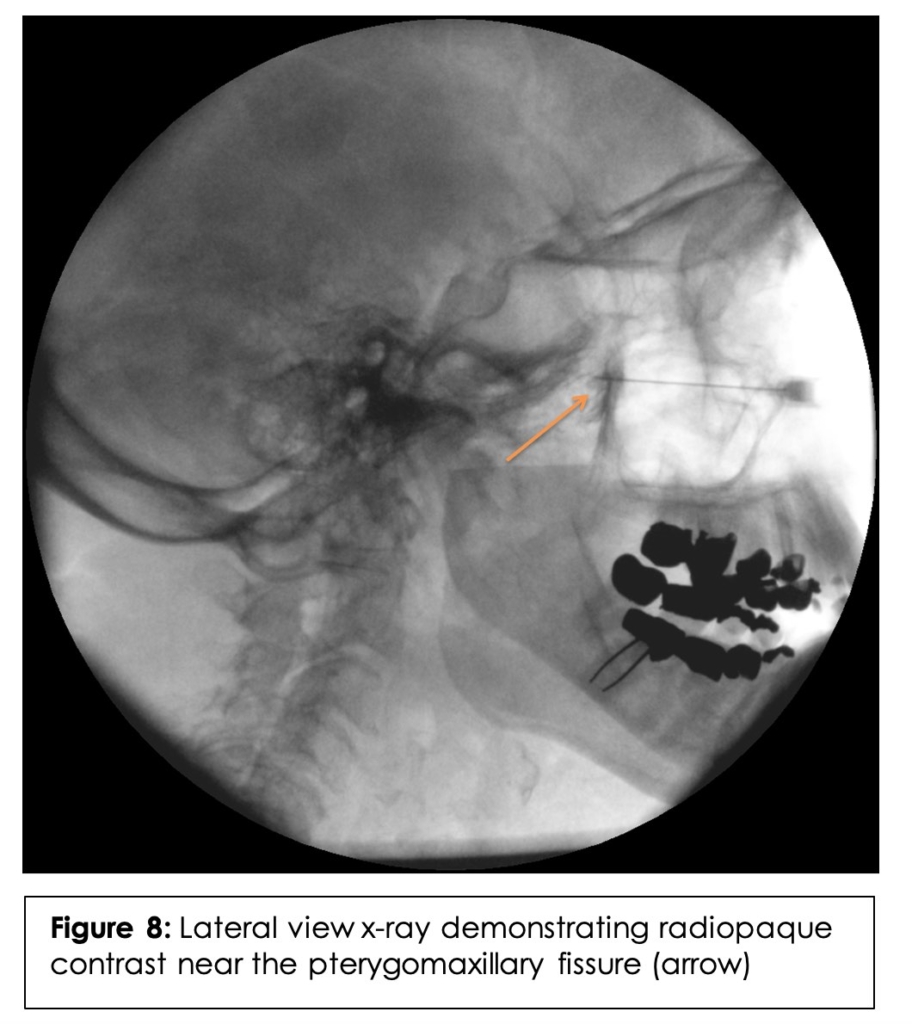
After the CT measurements were validated on the dissected side, the contralateral side, which was not dissected, was placed in a lateral decubitus position with the specimen’s nose parallel to the ground. The needle was injected using the same trajectory. A lateral cranial x-ray was obtained to ensure proper placement of the needle tip near the PMF. (Figure 6B) After confirmation of placement via x-ray, 1mL of radiopaque contrast was injected. A second lateral cranial x-ray was obtained which revealed contrast within the PMF, our primary target. (Figure 8)
Discussion
We present the first anatomical validation of SPG blocks in adult patients utilizing a suprazygomatic approach which reports the distance from the frontozygomatic angle to the PMF, needle angulations, and confirmation of target with radiopaque contrast injection.
While depth was increased in males and in patients greater than 40 years of age, we do not feel that this statistical difference is clinically significant. The average surface of the skin is approximately 1.65-1.78mm above the zygomatic arch which should be sufficient to prevent penetration of the needle into unwanted structures in the majority of patients in the proposed method described below. The measured inferior and posterior angles are generalizable to both genders and different age groups as there is no statistical difference when comparing these groups.
There may be instances when a SPG block is indicated but fluoroscopic or CT guidance is not available for use to perform a block via an infrazygomatic approach. Case reports have been demonstrated pain relief and no significant adverse events with suprazygomatic SPG blocks for patients with headache attributable to acute aneurysmal subarachnoid hemorrhage 11. These patients have intractable headaches and analgesic options are limited as opioids confound the neurological exam. In this circumstance the use of a suprazygomatic SPG block may be indicated when a fluoroscopically guided SPG block via an infrazygomatic approach is not feasible in the ICU. In addition, patients presenting with status migrainosis may also benefit from a suprazygomatic block in the ED or outpatient setting when a fluoroscopically guided SPG block is not available in a timely manner 3. In situations when a fluoroscopic or CT guided infrazygomatic SPG block are not available in a timely manner, the suprazygomatic approach may be a consideration.
Echaniz et al investigated suprazygomatic ultrasound (US)-guided maxillary nerve blocks through the PPF through the use of two cadavers 12. Utilizing US, they stated that the needle be placed at the frontozygomatic angle and advanced 14 degrees inferiorly and 15 degrees posteriorly and at a depth of 37mm to approximate inside the PPF. Using 1mL of injectate, inconsistent coverage of the maxillary nerve and SPG was obtained. They suggested that slightly more than 1mL be used to ensure effective analgesia. With the depth used in the technique, US guidance is likely needed to minimize the risk of injury of nerve structures, injury of the sphenopalatine artery, or penetration of the nasal cavity 13. US-guided maxillary nerve blocks utilizing a suprazygomatic approach have been described previously, but the distance from the surface of the skin to the PPF was not measured 14, 15. 15% of skulls have enlarged sphenoid processes or narrow PMFs, thus it might not be feasible to traverse the PMF to the PPF 16.
Other studies have performed suprazygomatic maxillary nerve blocks though mainly in pediatric patients 13, 14, 17. Most of these studies report various needle angulations and depths into the infratemporal fossa. Given the significant disparity in reported measurements, it is probable that, unless directly visualized, needle tips are not exactly in the location they are reporting. Variance in angle can be attributed to variation in patient size (neonate, infant, or adult). It is more likely that block efficacy is achieved through large volumes of local anesthetics. Large volumes allow spread of the injectate from the infratemporal fossa through the PMF to the PPF to affect either the maxillary nerve or SPG. Utilizing large volume of local anesthetic creates a risk that injectate can spread into nearby structures such as the orbit, or skull base. Injections are routinely between 2-5mL 14, 16, 17. A 5mL injection in the PPF spread backwards into the infratemporal fossa and above the temporal muscle, affecting branches of both the maxillary and mandibular nerves 12. Despite the large volume of injection directly into the PPF, further dissection revealed that injectate was not found to have spread into the orbit, nasal cavity, or cranial fossa 12.
One of the main strengths of using the approach presented in this study is that there appears to be no significant risk of penetration of the base of the skull or orbit 13, 15, 17. Strength of this study also lies in the large sample size of patients. By utilizing a large sample size, a more precise depth and angulation could be measured which will hopefully translate into a consistent patient response. These patients are representative of a wide range of age and weight as well. Additionally, with the calculated depth in this study, the risk of penetrating the maxillary artery is reduced as it lies ventrally and inferiorly to the maxillary nerve 13, 18. Even if it is injured, it is suggested that bleeding of the sphenopalatine artery into the infratemporal fossa would likely just result in mild hematoma formation and would not be life threatening 10. With the suprazygomatic approach described sedation and imaging with fluoroscopy may not be required.
The main limitation of this study is that the proposed depth and angles may not universally apply to all patients due to anatomical differences as evidenced by the significant range in all measured variables. Because of this, it is recommended that a larger volume of injectate be used to ensure adequate spread to the SPG. While larger volumes can lead to unnecessary complications, it is likely that unwanted side effects of local anesthetics will be transient 3, 19. Another limitation of this study is that the cranial CT measurements were created using Caucasians of Russian origin and therefore limits its applicability to a more diverse ethnic cohort, i.e., Asian, Indian, or of African descent.
Based on this study, a depth of 25mm (1 inch) at an inferior angle of 6 degrees and a posterior angle of 27 degrees should be sufficient to reach just outside the PMF if starting at the frontozygomatic angle in the majority of patients. However, while this angle may be generalizable, there is a significant minority of patients in which this may not work given the large range of angulation. In some patients, the shallow angle may lead to contact with the greater wing of the sphenoid as opposed to entering the infratemporal fossa. Redirection of the needle would be required which would likely lead to increased patient discomfort. For this reason, US guidance using this method may be needed to identify the frontozygomatic angle in order to deliver consistent results in a wide variety of patients.
Conclusion
Our findings suggest that using the frontozygomatic angle as an anatomical landmark, along with an approximation of the calculated inferior and posterior angulation for needle trajectory in this study, and a needle depth of 1 inch would be the optimal approach to perform suprazygomatic SPG blocks via the PMF that would be safe and effective on an anatomical basis. This method is generalizable and can be used in different age groups and genders. In special patient populations and circumstances, when fluoroscopic guidance is not readily available to perform a SPG block via an infrazygomatic approach, the suprazygomatic approach may be an option to provide pain relief. Further studies are needed to determine if image guidance is required to identify anatomical landmarks and guide needle trajectory in the clinical setting.
References
- Piagkou M, Demesticha T, Troupis T, et al. The pterygopalatine ganglion and its role in various pain syndromes: from anatomy to clinical practice. Pain Pract. 2012 Jun;12(5):399-412. PubMed CrossRef
- Osborn AG. Radiology of the pterygoid plates and pterygopalatine fossa. AJR Am J Roentgenol. 1979 Mar;132(3):389-394. PubMed CrossRef
- Mehta D, Leary MC, Yacoub HA, et al. The Effect of Regional Anesthetic Sphenopalatine Ganglion Block on Self-Reported Pain in Patients With Status Migrainosus. 2019 Jan;59(1):69-76. PubMed CrossRef
- Fontaine D, Santucci S, Lanteri-Minet M. Managing cluster headache with sphenopalatine ganglion stimulation: a review. J Pain Res. 2018 Feb;11:375-381. PubMed CrossRef
- Maizels M. and Geiger AM. Intranasal lidocaine for migraine: a randomized trial and open-label follow-up. 1999 Sep;39(8):543-551. PubMed CrossRef
- Crespi J, Bratbak D, Dodick D, et al. Measurement and implications of the distance between the sphenopalatine ganglion and nasal mucosa: a neuroimaging study. J Headache Pain. 2018 Feb;19(1):14. PubMed CrossRef
- Narouze S. Topical intranasal lidocaine is not a sphenopalatine ganglion block. Reg Anesth Pain Med. 2020 Dec 15;rapm-2020-102173. PubMed CrossRef
- Dziadzko MA, Heritier F. Suprazygomatic Access for Continuous Bilateral Mandibular Nerve Block for Pain and Trismus Relief in the Tetraplegic Patient. J Oral Maxillofac Surg. 2016 Oct;74(10):1947 e1-5. PubMed CrossRef
- Poore TE, Carney MT. Maxillary nerve block: a useful technique. J Oral Surg. 1973 Oct;31(10):749-755. PubMed
- Radder K, Shah A, Fatima S, et al. Efficacy and feasibility of frontozygomatic angle approach for extra oral maxillary nerve block in oral surgery: a descriptive clinical trial. J Maxillofac Oral Surg. 2014 Sep;13(3):231-237. PubMed CrossRef
- Melinosky C, Mehta D. Sphenopalatine Ganglion Blockade as a Novel Treatment for Aneurysmal Subarachnoid Hemorrhage Associated Intractable Headache. Neurocrit Care. Nov 2019:S258. CrossRef
- Echaniz G, De Miguel M, Merritt G, et al. Bilateral suprazygomatic maxillary nerve blocks vs. infraorbital and palatine nerve blocks in cleft lip and palate repair: A double-blind, randomised study. Eur J Anaesthesiol. 2019 Jan;36(1):40-47. PubMed CrossRef
- Captier G, Dadure C, Leboucq N, et al. Anatomic study using three-dimensional computed tomographic scan measurement for truncal maxillary nerve blocks via the suprazygomatic route in infants. J Craniofac Surg. 2009 Jan;20(1):224-228. PubMed CrossRef
- Stajcic Z, Todorovic L. Blocks of the foramen rotundum and the oval foramen: a reappraisal of extraoral maxillary and mandibular nerve injections. Br J Oral Maxillofac Surg. 1997 Oct;35(5):328-333. PubMed CrossRef
- Bouzinac A, Tournier JJ, Dao M, Delbos A. Ultrasound-guided maxillary nerve block in adults: feasibility and efficiency for postoperative analgesia after maxillary osteotomy. Minerva Anestesiol. 2014 Jul;80(7):860-861. PubMed
- Stojcev LS, Gacic B, Popovic N, Stajcic Z. Anatomical study of the pterygopalatine fossa pertinent to the maxillary nerve block at the foramen rotundum. Int J Oral Maxillofac Surg. 2010 May;39(5): 493-496. PubMed CrossRef
- Prigge L, van Schoor AN, Bosman MC, Bosenberg AT. Clinical anatomy of the maxillary nerve block in pediatric patients. Paediatr Anaesth. 2014 Nov;24(11):1120-1126. PubMed CrossRef
- Mesnil M, Dadure C, Captier G, et al. A new approach for peri-operative analgesia of cleft palate repair in infants: the bilateral suprazygomatic maxillary nerve block. Paediatr Anaesth. 2010 Apr;20(4):343-349. PubMed CrossRef
- Walker M, Drangsholt M, Czartoski TJ, Longstreth WT. Dental diplopia with transient abducens palsy. 2004 Dec;63(12): 2449-2450. PubMed CrossRef
Declarations
Conflict of Interest Statement
Dev G. Mehta reports no conflicts of interest.
Robert Gurianov declares no conflicts of interest.
Victor A. Gombolevskiy declares no conflicts of interest.
Sergey Morozov declares no conflicts of interest.
Carrie E. Robertson reports that she has served on the advisory board for Lundbeck, Biohaven, Amgen, Impel Neuropharma, and Eli-Lily. She also receives honoraria as an author from UpToDate.
Narayan R. Kissoon reports that he receives honoraria as an author from UpToDate and serves on the advisory board for Bright Mind Biosciences, Ltd.
Nirusha Lachman declares no conflicts of interest.
Sebastian Cotofana declares no conflicts of interest.
Funding
Funding for this study was provided by the Mayo Clinic Neurology Research Committee.
Acknowledgement
We would like to thank Kamal Makhmud M.D. MEDLAZ Clinic, Moscow, Russia for helping with radiology data analysis.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
