Author: Vasudha Goel MBBS * 1, Samer Narouze MD PhD 2
Author Affiliation:
1 Department of Anesthesiology, University of Minnesota, Minneapolis, MN
2 Center for Pain Medicine, Western Reserve Hospital, Cuyahoga Falls, OH
Research Support: Support was provided solely from institutional and/or departmental sources.
Competing Interests: The author/s declare no competing interests.
Issue: 02.07
DOI: doi.org/10.30756/ahmj.2020.02.07
Received: March 26, 2020
Revised: April 19, 2020
Accepted: April 20, 2020
Published: May 4, 2020
Recommended Citation: Goel V, Narouze S. Glossopharyngeal Neuralgia: An Approach to Diagnosis and Management. Ann Head Med. 2020;02:07. DOI: 10.30756/ahmj.2020.02.07
Glossopharyngeal neuralgia is an uncommon facial syndrome with significant deleterious effect on the quality of life. The glossopharyngeal nerve is predominantly a sensory nerve with a limited number of motor and autonomic fibers. The central causes of glossopharyngeal neuralgia are commonly treated with neurosurgical interventions. Medical therapy and nerve blocks are predominantly used to treat peripheral causes of glossopharyngeal neuralgia. In this review article, we present clinical vignettes and describe practical aspects of intra-oral, extra-oral, and peripheral techniques to block the glossopharyngeal nerve. The glossopharyngeal nerve blocks should be performed in a monitored setting due to the potential for adverse complications.
Introduction
Facial pain syndromes are commonly associated with a significant effect on a patient’s quality of life1. Glossopharyngeal neuralgia (GN) refers to a pain syndrome affecting mainly structures in the sensory distribution of the cranial nerve IX 2. Patients with GN have episodic sharp, stabbing, severe unilateral pain in the posterior region of the tongue, tonsillar fossa, pharynx, angle of the jaw, or occasionally the inner ear. The symptoms often arise due to activities resulting in sensory stimulation in the distribution of the Glossopharyngeal nerve (GNv). The most common activities are chewing, swallowing, coughing, talking, and yawing 3. Rarely patients can present with arrhythmias and syncope4. In this review, we describe the anatomy, clinical features, diagnostic work, and non-surgical therapies for the management of GN. In particular, we will focus on the management of post-tonsillectomy pain and Eagle’s syndrome.
Anatomy
The GNv is predominantly a sensory nerve with some preganglionic parasympathetic fibers and motors fibers (Figure 1).
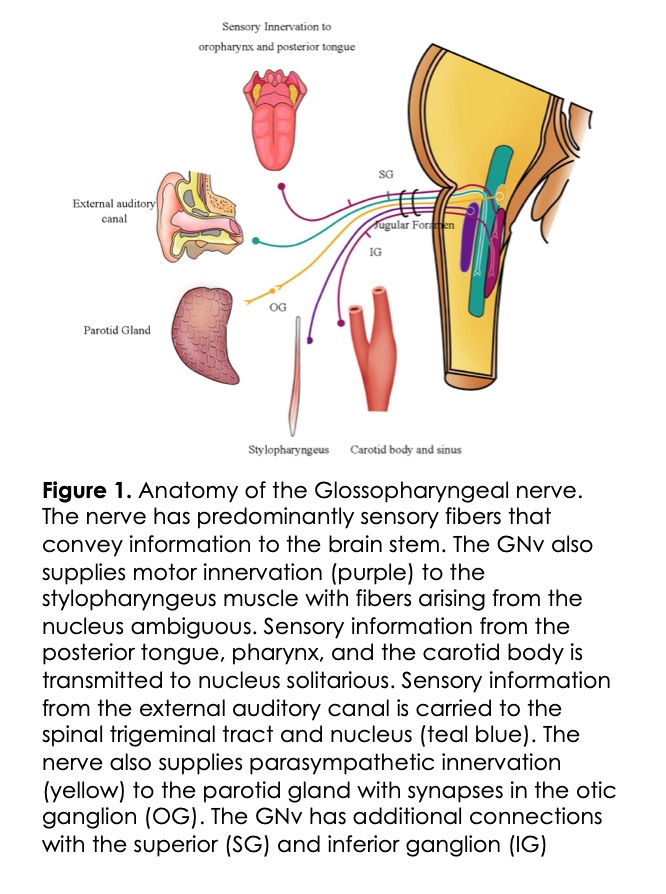
Sensory Component: The afferent or sensory fibers of the GN transmit information from areas of the mouth and pharynx, middle ear, and posterior one-third of the tongue and end in the trigeminal sensory nucleus. Special visceral afferent fibers (lingual nerve) carry signals from the taste buds of the posterior third of the tongue, which terminate in the nucleus solitarius. The sensory information mediates the swallowing and gag reflex in communication with the nucleus ambiguous and hypoglossal nucleus. These sensory fibers (nerve of Hering) also carry information from the chemoreceptors in the carotid body and baroreceptors in the carotid sinus. GNv also carries sensory information from the middle ear, mastoid through the tympanic branch, or Jacobson’s nerve5.
Motor Component: The GNv has limited motor fibers arising from the nucleus ambiguous, which innervate only the stylopharyngeus muscle involved in swallowing.
Autonomic Component: Preganglionic parasympathetic fibers of the GNv start in the inferior salivatory nucleus and synapse with the postganglionic fibers in the Otic ganglion which provide secretomotor fibers to the parotid gland.
Anatomical Course: GNv originates lateral to the olive in the rostral medulla as small rootlets, and then courses forward and laterally until it exits through the jugular foramen along with the vagus and spinal accessory nerves where it passes in-between the internal jugular vein (IJV) and the internal carotid artery (ICA). It descends anterior to the ICA and courses medially behind the styloid process where is starts to course away from the vagus nerve as it continues into its terminal branches. This extracranial course is along the posterior surface of the stylopharyngeus muscle. The nerve courses to the lateral surface eventually exiting through the gap between the superior and middle constrictors reaching the posterior surface of the tongue in the proximity of the palatine tonsil5.
The carotid sinus nerve branches off the GNv as it exits the jugular foramen. Through its course, it has complex communication with the vagus nerve and sympathetic nerves.
Epidemiology
GN is a rare neuralgia syndrome, and it accounts for approximately 1% of all cranial neuralgias. The population-level incidence is around 0.7/100000 individuals 6. The prevalence is similar among males and females, and the incidence increases with age. Most patients have unilateral pain (88%) and have spontaneous remission (76%) during follow up. GN is more common on the left side, while trigeminal neuralgia is more common on the right side 7.
Anatomical Sites Associated With GN
The GNv can be affected by lesions affecting the neuronal output from the brain before its origin in the brain stem, lesions affecting the nerve itself, extrinsic pressure from structures along the course, and by disease processes in the nearby structures (Table 1). Multiple sclerosis is one of the causes that may affect the GNv prior to its origin in the brain. Vascular compression, intracranial space-occupying lesions, posterior fossa and cervical malformations, and Eagle’s syndrome; are examples of disease states affecting the nerve due to extrinsic pressure. Sjogren and other autoimmune diseases can lead to neuritis and GN. Peritonsillar, intra-oral infections, and other cancers of the mouth are other peripheral causes of GN. While the cause in most patients may remain unknown (idiopathic), most patients are treated with medical therapy, and neurosurgical intervention may be offered to patients with intrinsic causes of pressure prior to the exit at the jugular foramen.
Pathophysiology
The anatomical course of the nerve and its relationships explains symptoms in secondary causes of GN. Most cases do not have an identifiable cause of neuropathy and are termed idiopathic GN. Any inflammation, infection, or compression from the nearby structures may result in hyperexcitability of the nerve resulting in symptoms. Vascular compression of the nerve roots can lead to demyelination and ephaptic communication. The abnormal impulses due to repeated activation of the afferent neurons modulate hyperexcitability in the central nuclei 8.
Differential Diagnosis
Hyperactivity associated with other cranial nerves and myofascial Pain Dysfunction Syndrome are the main disorders that can be confused with GN 9. Various hyperactive cranial nerve syndromes include trigeminal neuralgia (CN V), superior laryngeal neuralgia (CN X), and intermediate nerve neuralgia (CN VII) 2. The diagnosis of GN is confirmed when symptoms improve with nerve block at the jugular foramen or with topical anesthesia of the pharynx.
Management
GN should be suspected based on clinical history and characteristics of pain associated with the episodes. A focused physical exam evaluating the potential causes of GN may provide additional clues. High-resolution Computed Tomography (CT) or Magnetic Resonance imaging of the brainstem may be needed to identify compressive causes and demyelinating lesions, leading to GN. Radiography or high-resolution CT imaging may reveal an elongated styloid process suggestive of Eagle’s syndrome as a cause for GN. Patient education, along with pharmacotherapy, is the mainstay of treatment for GN. In selected cases, nerve blocks and additional surgical interventions are warranted (Table 2).
In the paper below, we focus on recent advances in the management of GN due to Eagles’ syndrome, peritonsillar, and intra-oral lesions among patients who failed conservative therapy.

Clinical Vignette
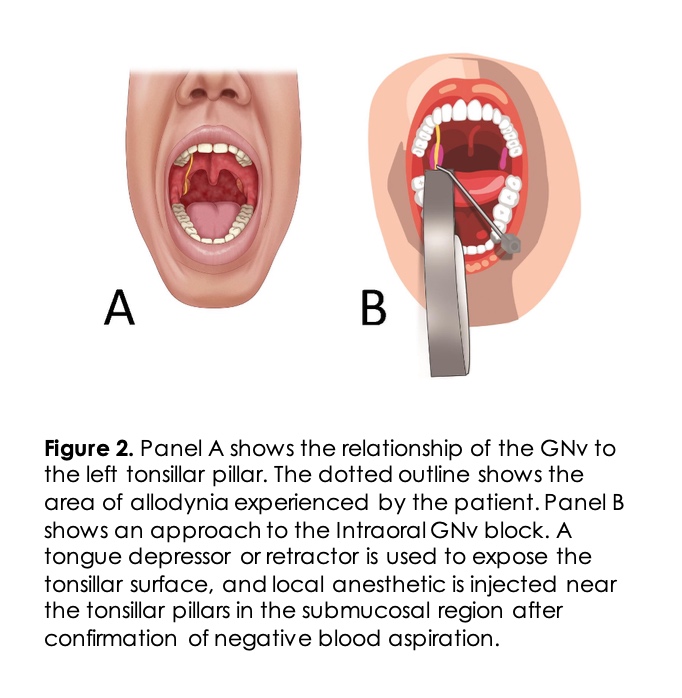
A 56-years-old female complained of chronic left-sided throat pain that was initially started in the recovery room after a tonsillectomy surgery under general anesthesia five years prior to this presentation. The pain was localized to the left base of the tongue, left tonsil area, and the soft palate with a sensation of swelling of these structures. She woke up from surgery with burning pain and a raw sensation in the interior of her throat that has not changed over the past five years. Mostly her pain increased with eating, especially spicy foods, and swallowing movements in her oropharynx. Her physical examination was within normal limits with evidence of scarring of the tonsillar fossa as expected after tonsillectomy surgery. She had allodynia in the areas shown in Figure 2A. After her tonsillectomy surgery, the patient had a prolonged empiric course of antibiotics under the guidance of an infectious disease specialist. She could not tolerate gabapentin because of side effects. She had tried pregabalin, duloxetine, baclofen, and tricyclic antidepressants without success.
GN was suspected, and a GNv block was suggested to confirm the diagnosis. She was interested and extremely anxious about the invasive approach to the glossopharyngeal nerve, especially in the light that she had not had much success with any previous treatments. A non-invasive intra-oral approach to GNv block was attempted for diagnostic purposes. After informed consent, the patient was placed in a recliner chair with monitors attached. A cotton pledget soaked in 4% lidocaine was held with angled-forceps ensuring the pledges extended beyond the metal tip to avoid injury. A string was wound around the pledges to avoid dislodgement. The soaked pledges were applied to the left side base of the tongue at the inferior portion of the tonsillar fossa in the area where the glossopharyngeal nerve crosses the posterior and anterior tonsillar pillars and is superficial. The soaked pledges were applied for 1-2 minutes, after which the patient described a numb sensation in the left side of the throat and reduction in pain in the recovery area. On follow up after three weeks, the patient reported a significant reduction in pain. She reported an overall 40% reduction in pain in the left throat. She continued to have reduced pain for about one month, at which time a spicy meal triggered her pain. She similarly underwent a second nerve block and continued to have improved pain control for over three months.
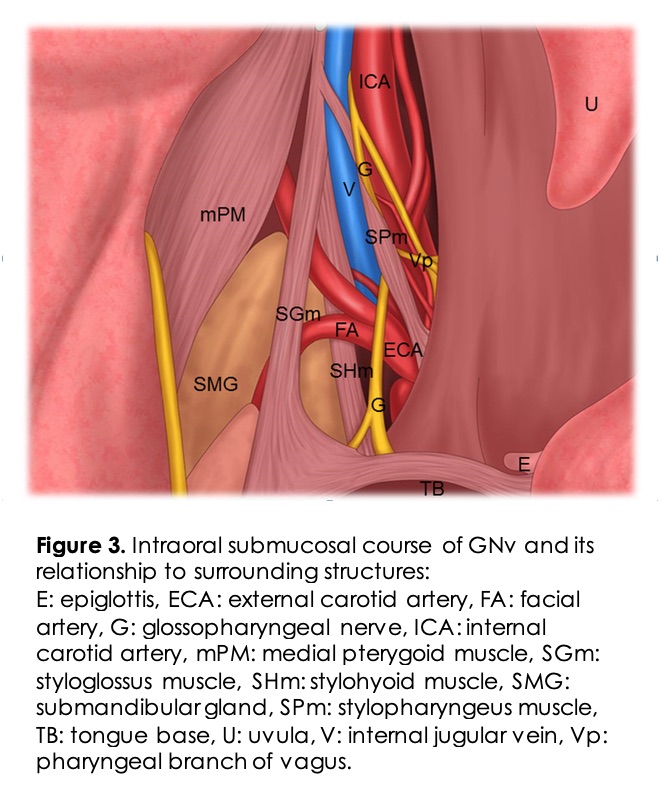
Intra-oral GNv Block: GN is a rare diagnosis that can occur uncommonly post-tonsillectomy, as in our patient. A GNv block can be challenging for pain physicians as it is not commonly performed because of the rarity of this neuralgia. Noninvasive intra-oral GNv block may be included as the first step in the diagnosis. GNv block using cotton pledges soaked in local anesthetics has been used by anesthesiologists for the management of difficult airways to limit gag reflex. The GNv can be blocked intra-orally in the vicinity of the tonsillar pillars (intra-oral/ premolar approach) and is considered safe. The patient is in a seated or supine position with a wide-open mouth, and the tongue is retracted medially and downward using a tongue depressor or a laryngoscope blade 10. A bent needle or a 22-25G straight needle is used to aspirate and subsequently inject 1-2ml local anesthetic medication in the submucosal plane near the tonsillar pillar (Figure 2 B). Relevant intraoral anatomy is shown in Figure 3. The needle should be rotated 90 degrees, and respiration attempted to minimize the risk of vascular injection 11. Awake fiberoptic intubation requires a block of the GNv, superior laryngeal nerve, and translaryngeal nerve blocks. Recent reports of ultrasound guidance for nerve block along with local anesthetic spray in the oropharynx demonstrated successful nerve block of the airway even in an acidic environment like infected tissue 12. Intraoral GNv block using lidocaine spray with video laryngoscopy has been described for pharyngeal anesthesia in a patient with severe nasal bleeding 13.
Clinical Vignette
A 45-year-old male presented with sharp neck pain with radiation to the ear and throat. He had a foreign body sensation and difficulty swallowing in the throat for the past year. His past medical history was otherwise unremarkable. A high-resolution CT scan showed an elongated styloid bone confirming the diagnosis of Eagle’s syndrome. After an unsuccessful trial of gabapentin, the patient opted to undergo a fluoroscopic-guided GNv block. The patient had improvement in clinical symptoms and underwent a repeat injection at three months.
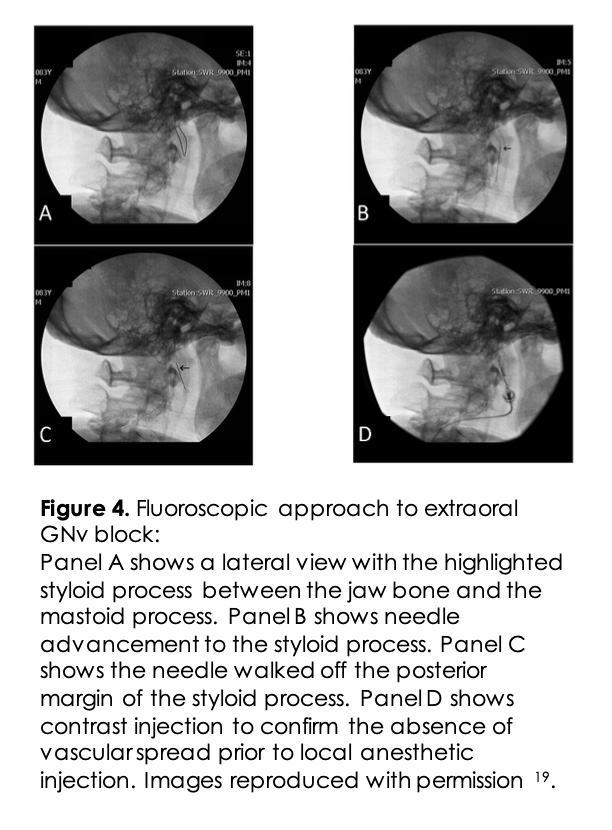
Extra-oral GNv Block: The percutaneous extra-oral GNv block is most often performed using imaging guidance. Approaches using fluoroscopy and ultrasound guidance have previously been described 14. For the fluoroscopic approach, the patient is placed supine, and the head is turned to the unaffected side. Lateral fluoroscopic view is obtained to view the angle of the jaw and the mastoid process (Figure 4). The midpoint of an imaginary line between the two points is an anatomic landmark for the styloid process. The skin is prepped with an antiseptic solution and is anesthetized with lidocaine. A 25G long needle is advanced until the styloid process is contacted. Once the styloid process is touched, the needle is walked-off posteriorly. Negative aspiration is confirmed before the injection of real-time contrast to rule out an intravascular spread. Then 2-3ml of local anesthetic with or without non-particulate steroids is injected.
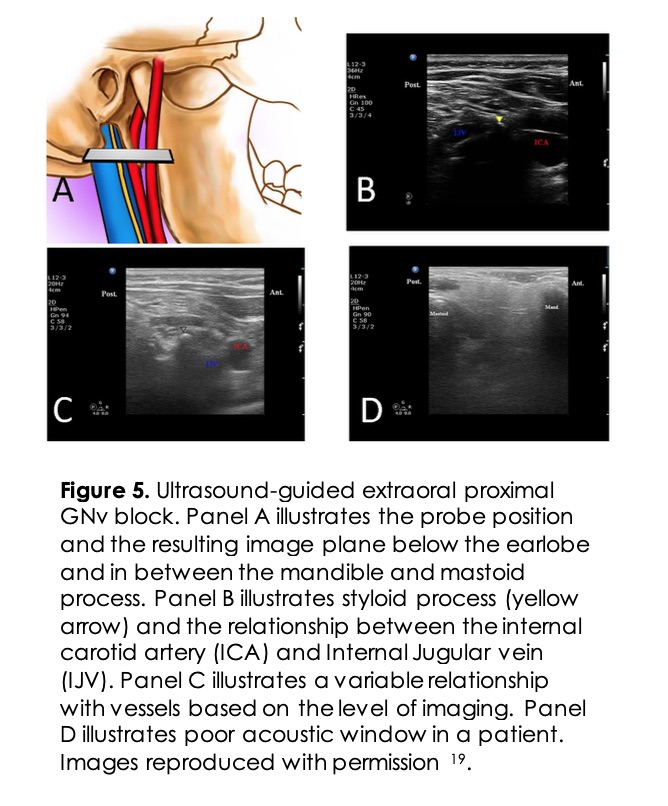
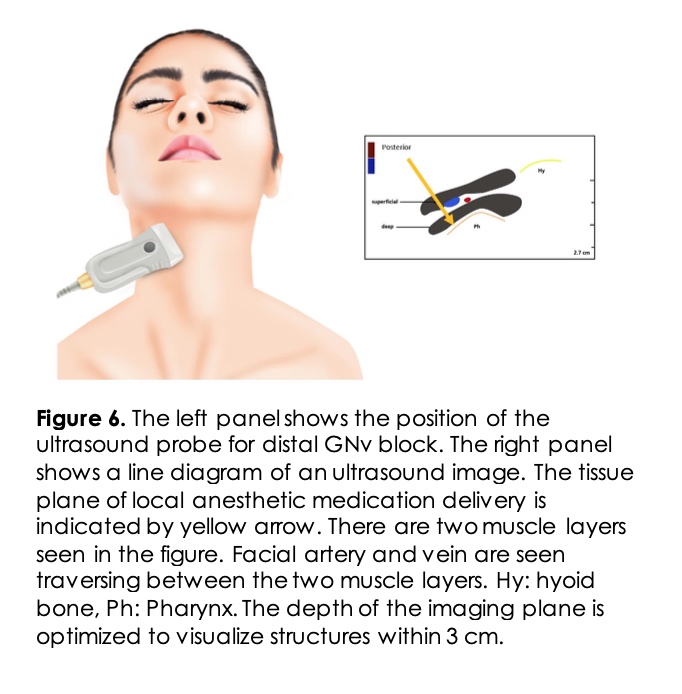
Ultrasound approaches use a linear probe placed below the patient’s ear lobe between the angle of the mandible and the mastoid process (Figure 5). The patient’s neck is prepared in a sterile fashion and turned to the unaffected site. The skin is anesthetized with local anesthetic solution. The styloid bone is visualized as a hyperechoic area medial to the mastoid process. When the styloid process is not identified, the internal carotid artery can be followed up to the ear lobe. The internal carotid artery is used as a landmark with a probe at the level of the ear lobe. The needle is directed posteriorly, and after a negative aspiration test, 2-3ml of local anesthetic with or without steroids is injected 15. Newer techniques of GNv block more distally near the pharyngeal wall have been described. The pharyngeal wall and the horn of the hyoid bone are identified, and the local anesthetic is injected in the parapharyngeal space16 (Figure 6). This technique allows real-time visualization and at the same time avoids damage to the vascular structures and other nerve blockade.
Precautions and Procedural Complications
Due to the close proximity of the GNv to major vascular structures and other cranial nerves, any interventions should be performed in a monitored setting with access to resuscitative equipment. Intravascular injections can lead to seizures and hemodynamic collapse. Local vascular injury in the neck can lead to hematoma formation and compression of the nearby structures. The risk of infection should be weighed in patients with oral infections undergoing intra-oral block or immunocompromised patients undergoing extra-oral GNv block. Patients with prior neck radiation therapy or neck surgery will require additional precautions to avoid complications. Intra-oral GNv block has a potential for injection into the tonsillar artery. Dysphagia and dryness may occur due to interruption of the motor supply to stylopharyngeus muscle. Dysphonia can result from recurrent laryngeal nerve palsy. Temporary weakness of the tongue and shoulder may occur due to block of the hypoglossal and spinal accessory nerves. Vagal nerve blockade can result in reflex tachycardia, hypertension, arrhythmias, and may be associated with hemodynamic instability.
Outcomes
Most of the GNv block reports are either single cases or case series. Several randomized trials show the safety and efficacy of GNv block for patients undergoing tonsillectomy. 17,18
Conclusion
Glossopharyngeal neuralgia is a distinct syndrome associated with irritation or entrapment of the glossopharyngeal nerve (CN IX). Most cases do not have an identifiable cause. Patients are treated with medical therapy, interventional blocks in refractory cases, or with surgical treatment and neuromodulation. Both intraoral and extraoral GNbv block can be safely and effectively performed with a proper understanding of regional anatomy. Recent advances in ultrasound-guided distal block in the retropharyngeal space has the potential to minimize adverse events.
References
- Zakrzewska JM. Differential diagnosis of facial pain and guidelines for management. Br J Anaesth. 2013 Jul;111(1):95-104. PubMed CrossRef
- Blumenfeld A, Nikolskaya G. Glossopharyngeal neuralgia. Curr Pain Headache Rep. 2013 Jul;17(7):343. PubMed CrossRef
- Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018 Jan;38(1):1-211. PubMed CrossRef
- Hu TY, Kapa S, Cha YM, Asirvatham SJ, Madhavan M. Swallow-induced syncope: A case report of atrial tachycardia originating from the SVC. HeartRhythm Case Rep. 2015 Nov 17;2(1):83-87. PubMed CrossRef
- Wang C, Kundaria S, Fernandez-Miranda J, Duvvuri U. A description of the anatomy of the glossopharyngeal nerve as encountered in transoral surgery. Laryngoscope. 2016 Sep;126(9):2010-5. PubMed CrossRef
- Katusic S, Williams DB, Beard CM, et al. Incidence and clinical features of glossopharyngeal neuralgia, Rochester, Minnesota, 1945-1984. Neuroepidemiology. 1991;10(5-6):266-75. PubMed CrossRef
- Rushton JG, Stevens JC, Miller RH. Glossopharyngeal (vagoglossopharyngeal) neuralgia: A study of 217 cases. Arch Neurol. 1981 Apr;38(4):201-5. PubMed CrossRef
- Ferroli P, Franzini A, Farina L, et al. Does the presence of a pontine trigeminal lesion represent an absolute contraindication for microvascular decompression in drug resistant trigeminal neuralgia? J Neurol Neurosurg Psychiatry. 2002 Jan;72(1):122-3. PubMed CrossRef
- Khan M, Nishi SE, Hassan SN, et al. Trigeminal neuralgia, glossopharyngeal neuralgia, and myofascial pain dysfunction syndrome: An update. Pain Res Manag. Epub 2017 Jul 30. PubMed CrossRef
- Atlas G, Sifonios A, Otero J. Use of a laryngoscope, held sideways, as an aid in perforsming an intraoral glossopharyngeal nerve block. J Anaesthesiol Clin Pharmacol. 2013 Jan;29(1):129-30. PubMed CrossRef
- Rao S, Rao S. Glossopharyngeal nerve block: The premolar approach. Craniomax Traum Rec. 2018 Dec;11(4):331-332. PubMed CrossRef
- Krause M, Khatibi B, Sztain JF, et al. Ultrasound-guided airway blocks using a curvilinear probe. J Clin Anesth. 2016 Sep;33:408-412. PubMed CrossRef
- Komatsu M, Komasawa N, Yonehara S, Minami T. Efficacy of videolaryngoscope-guided glossopharyngeal nerve block in a patient with severe nasal bleeding. J Clin Anesth. 2018 Feb;44:18. PubMed CrossRef
- Nihei S, Sato J, Komatsu H, et al. The efficacy of sodium azulene sulfonate l-glutamine for managing chemotherapy-induced oral mucositis in cancer patients: A prospective comparative study. J Pharm Health Care Sci. 2018 Aug 13;4:20. PubMed CrossRef
- Maher T, Shankar H. Ultrasound-guided peristyloid steroid injection for eagle syndrome. Pain Pract. 2017 Apr;17(4):554-557. PubMed CrossRef
- Azman J, Pintaric TS, Cvetko E, Vlassakov K. Ultrasound-guided glossopharyngeal nerve block a cadaver and a volunteer sonoanatomy study. Reg Anesth Pain Med. 2017 Mar/Apr;42(2):252-258. PubMed CrossRef
- El-Hakim H, Nunez DA, Saleh HA, et al. A randomised controlled trial of the effect of regional nerve blocks on immediate post-tonsillectomy pain in adult patients. Clin Otolaryngol Allied Sci. 2000 Oct;25(5):413-7. PubMed CrossRef
- Park HP, Hwang JW, Park SH, et al. The effects of glossopharyngeal nerve block on postoperative pain relief after tonsillectomy: The importance of the extent of obtunded gag reflex as a clinical indicator. Anesth Analg. 2007 Jul;105(1):267-71. PubMed CrossRef
- Narouze SN. Interventional management of head and face pain. Nerve blocks and beyond. 1st New York: Springer, 2014. Chapter 6, Glossopharyngeal Nerve Block; p. 41-46. CrossRef
Contribution Statement
Vasudha Goel and Samer Narouze designed the review, drafted, and revised the paper. Vasudha Goel is a guarantor.
Disclosures
Dr. Samer Narouze is the current EIC for Annals of Headache Medicine. Dr Dmitri Souza was the acting EIC handling this manuscript.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: Samer Narouze MD is the Chairman of the Board and Founder of the American Interventional Headache Society (AIHS).
