Author: Michael A Levenson, BS 1, David I Levenson, MD, FACP, FACE * 2
Author Affiliation:
1 MD Candidate, Technion Faculty of Medicine, Haifa, Israel
2 Affiliate Assistant Professor of Integrative Medical Science, Florida Atlantic University College of Medicine, Boca Raton, FL
Research Support: Support was provided solely from institutional and/or departmental sources.
Competing Interests: The author/s declare no competing interests.
Issue: 02.08
DOI: doi.org/10.30756/ahmj.2020.02.08
Received: March 26, 2020
Revised: April 19, 2020
Accepted: April 20, 2020
Published: May 6, 2020
Recommended Citation: Levenson MA, Levenson DI. Insulin as a Potential Treatment in Selected Migraine Sufferers: Two Case Reports. Ann Head Med. 2020;02:08. DOI: 10.30756/ahmj.2020.02.08
Objectives
To review the change in headache frequency of two diabetic patients before and after initiation of insulin therapy, and to generate hypotheses regarding possible mechanisms underlying the potential link between headaches and glucose regulation.
Case Report
Hyperglycemia and hypoglycemia are both triggers for migraines. We present a series of two cases of women with diabetes who were started on insulin therapy in an attempt to reduce the frequency of their chronic migraine headaches. To our knowledge, this is the first such report in the literature. Both patients had glucose control at goal (A1c <6.5) and otherwise would not have warranted initiation of insulin. One patient had a 90% reduction in migraine frequency. The other had a 50% reduction in migraine frequency, and an 80-90% reduction in cluster headache frequency.
Conclusion
Fluctuations in glucose levels, rather than changes in insulin levels, are the likely triggers of the headaches seemingly associated with carbohydrate consumption. Judicious use of insulin in carefully selected patients may represent a novel method of preventing migraines.
Introduction
In addition to more classic stimuli, migraines may be triggered by high carbohydrate meals, prolonged fasting, and hypoglycemia. A ketogenic (low carbohydrate) diet has been shown to reduce migraine frequency, implying a potential causal relationship.1
Chronic migraines have been associated with insulin resistance, metabolic syndrome, and obesity.2 Even in non-obese subjects, higher levels of insulin resistance were associated with more frequent migraines and higher fasting glucose levels.3 In contrast, Fagherazzi et al showed a lower prevalence of medication-treated diabetes in migraine patients.4 Interestingly, in this cohort prevalence of migraines decreased from 22% to 11% in the 24 years prior to diagnosis of diabetes, implying a possible protective role for diabetes in frequency of migraine attacks.
Hypoglycemia has also been described as a trigger. Fagherazzi et al hypothesized that the rising sugar levels during the prediabetic state resulted in less hypoglycemia, and this could have been the reason for fewer migraines. Candan et al reports a case of migraines associated with postprandial (reactive) hypoglycemia in a non-diabetic.5 However, one cannot exclude a transient premonitory hyperinsulinemic/hyperglycemic phase (even within the normal glucose ranges).
We report two cases of diabetic patients who noted migraine headaches associated with excursions of glucose within the normal range (130-140 mg/dl). Both were empirically treated with insulin and had a dramatic improvement in the frequency of headaches. To our knowledge, these are the first such reports.
Both patients agreed to have their information published and were given a draft of the article to read prior to submission.
Case Report #1
A 35-year-old Bahamian woman presented with chronic migraines since age 12, and chronic cluster headaches since age 21. Triggers include scents as well as many different packaged or processed foods. Medical history and associated home medications include: pseudotumor cerebri (diagnosed age 16, on acetazolamide 500 mg three times daily), Addison’s disease (hydrocortisone 10 mg daily and dexamethasone 0.25 mg daily), Type 2 diabetes mellitus since age 33 (metformin 2000 mg daily and liraglutide 1.8 mg daily), empty sella syndrome and morbid obesity (Weight 265 lbs, Body Mass Index (BMI) = 41.5 kg/m2).
The headaches occurred predominantly postprandially, and when glucose >130 mg/dl. They would be less frequent or less intense when the patient restricted food or fasted. On the other hand, she sometimes developed reactive hypoglycemia (glucose <70 mg/dl) 4-5 hours after the last meal, triggering a migraine. Gabapentin 100 mg at the onset of a headache did not help, but codeine phosphate hemihydrate 30 mg and paracetamol 500 mg did offer some relief. Her A1c was 5.4, with occasional hypoglycemia. Liraglutide was discontinued (because of side effects).
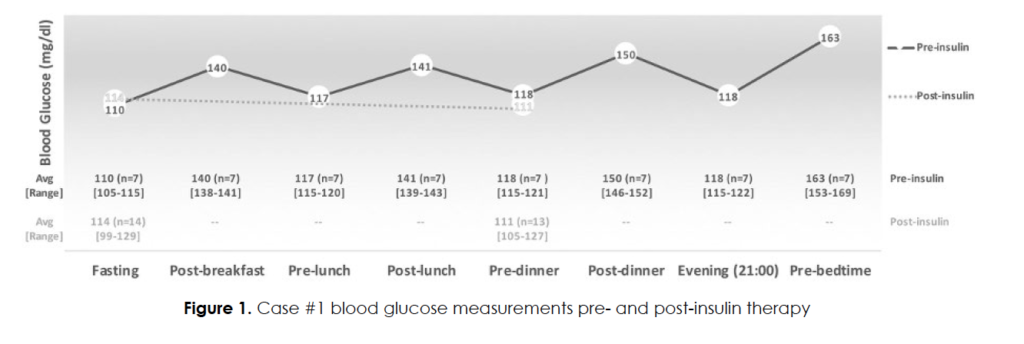
Her headaches occurred daily. Figure 1 shows her glucose excursions during the week prior to initiation of insulin. Her fasting glucose levels were only mildly elevated (average = 114 mg/dl), and her postprandial excursions averaged 150 mg/dl after dinner and 163 mg/dl after a bedtime snack. Noting the association of food intake (specifically carbohydrates) as a trigger, she was empirically started on insulin aspart 2-3 units (approximately 0.02 units per kg) prior to meals, working up to 60 units daily in divided doses (total daily dose 0.5 unit/kg/day). She was very insulin resistant, with one unit of insulin lowering her glucose by only 3-5 mg/dl. During a representative two-week period while on insulin, fasting glucose levels averaged 114 mg/dl and pre-dinner averaged 111 mg/dl. As expected, the premeal glucose levels were not significantly different from before initiating (only) short-acting insulin. In contrast, the postprandial glucose excursions were reduced (patient reported, data not shown) and corroborated by an excellent A1c = 5.3. Self-reported migraine headaches improved by 50%, and her cluster headaches improved by 80-90%. She reported better sleep and overall feeling better. She does still experience periods of low glucose levels, but the associated headaches are less intense.
Case Report #2
A 60-year-old Caucasian woman with a history of gestational diabetes had her first migraine at age 37, six hours after the birth of her daughter. She had gestational diabetes during that pregnancy. For the first three years she had visual auras, but more recently her migraines are associated with unilateral leg weakness, arm numbness and, in severe instances, even slurred speech. Additionally, there are multiple classic triggers, including sunlight, stress, and certain foods (chocolate, alcohol, and cheese). She uses naproxen, ibuprofen and acetaminophen/butalbital/caffeine for management of the headaches. Headaches occur as often as 25 days a month. She was subsequently diagnosed with Type 2 diabetes mellitus at age 60 with Fasting Blood Sugar (FBS) = 128 mg/dl and A1c = 6.5. Her weight is 124 lbs (BMI = 21.3 kg/m2).
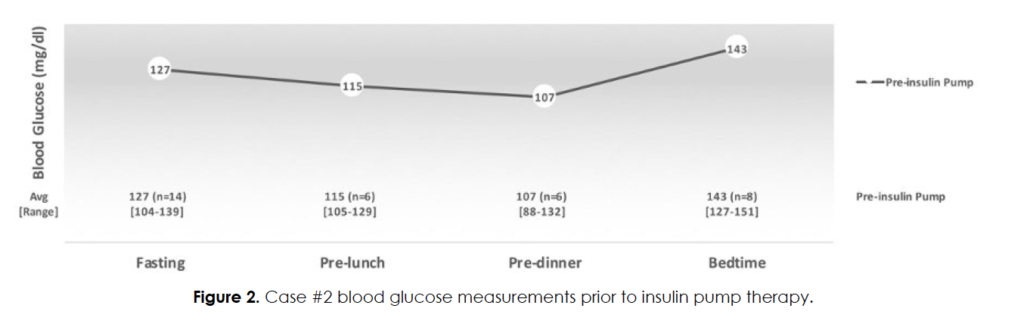
Attempts to better control her blood glucose levels with pioglitazone (worsening of headaches) and metformin (dizziness) failed. She monitored her blood glucose levels carefully and noted that headaches began when postprandial glucose levels exceeded 140 mg/dl or fasting glucose levels exceeded 120 mg/dl. Figure 2 shows her glucose averages during the month prior to initiation of insulin (while on metformin 500 mg daily). She would typically fast all day (to avoid triggering a migraine) and would consume her entire daily caloric intake with dinner and evening snacks. She was initially given 1 – 1.5 units insulin lispro prior to meals with a self-reported 90% improvement in frequency of headaches.
Continuous glucose monitoring confirmed dawn phenomenon, postprandial glucose excursions and no hypoglycemia. She was insulin sensitive, and one unit of insulin resulted in an average blood glucose reduction of 40 mg/dl. In an effort to get the morning sugars reliably less than 100 mg/dl and not risk low sugars during the daytime, she was started on an Omnipod® insulin pump. Insulin lispro was diluted 1:10 with normal saline prior to filling the reservoir. The effective basal insulin delivery rates were 0.21 units per hour overnight and a negligible
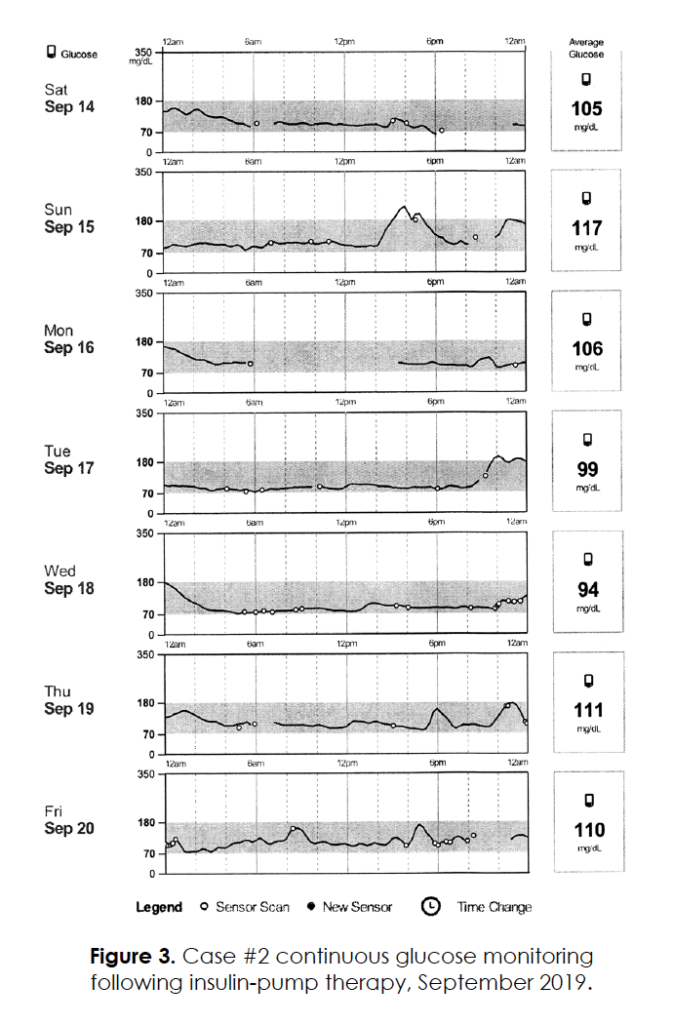
0.005 units per hour during the daytime. An additional 1-2 units of insulin were administered with each meal, and the patient had excellent glucose control (A1c = 6.0) with no documented hypoglycemia. Continuous glucose monitoring while on the insulin pump (Figure 3) confirmed absence of dawn phenomenon; morning glucose levels averaged 110 mg/dl, and overall 24-hour glucose levels also averaged 110 mg/dl. Since initiation of insulin, she has experienced a self-reported 90% reduction in headache severity/frequency; her occasional residual headaches are often associated with miscalculating carbohydrate meal size or forgetting to take a bolus of insulin.
Discussion
We present two cases of women with chronic migraines whose symptoms dramatically improved following insulin administration. The first patient was morbidly obese, taking glucocorticoids and was relatively insulin resistant. The second patient, in contrast, was thin and extremely insulin sensitive. They both noted headache onset when postprandial blood glucose levels reached the upper ranges of normal (130-140 mg/dl). The second case also noted headaches when fasting glucose levels were greater than 120 mg/dl, and near absence of headaches when glucose levels were below that.
In terms of hypoglycemia, the first case noted headaches when the glucose level fell below 70 mg/dl; this was present both before and after initiation of insulin therapy. The second case never had documented hypoglycemia. Both subjects consumed relatively low carbohydrate meals, and the second case actually expanded her food choices once on insulin.
As mentioned above, the mechanisms underlying chronic migraine headaches are undoubtedly complex and likely multifactorial. Here we present three non-mutually exclusive hypotheses regarding the role/mechanism of carbohydrate consumption in triggering migraines. First, by elevations in blood glucose levels. Second by elevations in insulin (or other hormone) levels. Third, by a rebound reactive hypoglycemia.
In both patients, post prandial blood glucose levels >130-140 mg/dl (and fasting blood glucose levels >120 mg/dl in the second patient) were correlated with onset of migraines. For both patients, larger meals were more likely to trigger a migraine. Zhang et al reports a rise in plasma glucose levels in the aftermath of spontaneous migraines, likely reflecting hormonal or physiological changes of the migraine itself rather than intrinsic regulation of blood glucose levels.6 The mean time to glucose sampling was 7.6 hours after headache onset. Our study is trying to correlate the ambient glucose levels immediately prior to the onset of migraine and any role in triggering a migraine. We have no data to postulate changes in glucose levels following a migraine, certainly not 8 hours later. Nonetheless, Zhang et al also document an association of elevated blood glucoses and migraines.6
It is unlikely that fluctuations/increases in plasma insulin levels directly resulted in migraine symptoms. In both patients, insulin administration (fairly large doses with the first case and very small doses with the second case) resulted in a marked diminution in headache frequency and intensity. Most probably, the ambient plasma insulin levels were higher in these patients for the following two reasons. First, subcutaneous insulin avoids the degradation involved in first-pass hepatic metabolism. In rat models, compared to portal insulin delivery, subcutaneous insulin delivery results in higher plasma insulin levels and lower portal vein insulin levels.7 Consequently, compared to portal delivery of insulin, subcutaneous insulin delivery results in lower hepatic disposal of glucose and proportionally greater systemic glucose disposal.7 Second, as expected following exogenous insulin administration, the postprandial plasma glucose levels were lower in both patients. As such, it stands to reason that subcutaneous/exogenously administered insulin results in a higher degree of systemic hyperinsulinemia (and subsequently lower blood glucose levels) than endogenous insulin.
McCarthy et al describes five (of 48) polymorphisms in the insulin receptor gene that are associated with typical migraines.8 However, they showed that neither migraine status, disease severity, migraine frequency, nor the presence of particular migraine trigger factors (such as fasting) significantly affected 125I-insulin binding to the insulin receptor. Likely, insulin resistance was unaffected and that systemic insulin levels (not actually tested) would not have been affected by these single-allele polymorphisms even if they were present in the two aforementioned cases.
C-peptide has recently been shown to have metabolic effects, including enhancing glucose disposal.9 In theory it could be a trigger for migraines. C-peptide is secreted in an equimolar fashion with endogenous (but not exogenous) insulin. Administration of exogenous insulin would be expected to lower C-peptide levels. However, this is an unlikely mechanism, at least in case #2. Case #2 was extremely insulin sensitive, and likely had minimal C-peptide levels at any given time. Case #1 also had (reactive) hypoglycemia-induced migraines. However, these headaches occurred many hours after a meal when the C-peptide would be expected to be significantly degraded by then, since she had normal kidney function.
Thus, it is logical to presume that hyperinsulinemia, per se, or elevations in C-peptide levels are not likely to be triggers for migraines, at least in these two patients. However, there is an association of a higher incidence of insulin resistance in migraine patients.2 This could be an epiphenomenon, and merely a correlate for hyperglycemia, and/or reactive hypoglycemia.
Case #1 did have documented hypoglycemia, both before and after initiation of insulin. Case #2 has had no hypoglycemic episodes; she uses a continuous glucose monitor which would even pick up nocturnal hypoglycemia. Both patients are very pleased, expressing improved quality of life, and have elected to stay on insulin. The second patient reports: “I have my life back.”
Study limitations include the following: It was a small observational study of only two subjects. Insulin and C-peptide levels were not measured at the onset of headaches. Most of the data is based on patient reporting. The strength of these case reports is their interventional nature. Both patients reported that when they were less adherent with insulin or food choices, there was a higher frequency of headaches; conversely, headache control was markedly improved with better adherence/glucose control.
To our knowledge, this is the first published report of using insulin specifically as a treatment to prevent migraines.
Hyperglycemia, per se, was likely the trigger for headaches in both patients.4 In the first case, there were innumerable episodes of hypoglycemia-induced headaches which began many hours after a meal, with no documented antecedent hyperglycemia. It would seem hypoglycemia is also an independent trigger for migraines. Thus, the best fit hypothesis would be that fluctuations in glucose per se, and not insulin or C-peptide levels, are potential triggers for migraines.
For selected patients with migraines who associate their headaches with carbohydrate consumption, it may be beneficial to perform glucose monitoring and see if there is an association with their headaches and mild excursions of glucose (even within the “normal range”). An empiric trial of insulin (or possibly oral antidiabetic therapy) might be helpful in dramatically improving their quality of life.
References
- Di Lorenzo C, Coppola G, Bracaglia M, et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J Headache Pain. 2016;17:58. PubMed CrossRef
- Fava A, Pirritano D, Consoli D, et al. Chronic migraine in women is associated with insulin resistance: a cross-sectional study. Eur J Neurol. 2014 Feb;21(2):267-272. PubMed CrossRef
- Siva ZO, Uluduz D, Keskin FE, et al. Determinants of glucose metabolism and the role of NPY in the progression of insulin resistance in chronic migraine. Cephalalgia. 2018 Oct;38(11):1773-1781. PubMed CrossRef
- Fagherazzi G, El Fatouhi D, Fournier A, et al. Associations Between Migraine and Type 2 Diabetes in Women: Findings From the E3N Cohort Study. JAMA Neurol. 2019 Mar 1;76(3):257-263. PubMed CrossRef
- Candan FU. EHMTI-0229. A case of migraine like headache with postprandial hypoglycemia treated with lifestyle changing. J Headache Pain. 2014;15(Suppl 1):G39. CrossRef
- Zhang DG, Amin FM, Guo S, et al. Plasma Glucose Levels Increase During Spontaneous Attacks of Migraine With and Without Aura. Headache. 2020 Apr;60(4):655-664. PubMed CrossRef
- Farmer TD, Jenkins EC, O’Brien TP, et al. Comparison of the physiological relevance of systemic vs. portal insulin delivery to evaluate whole body glucose flux during an insulin clamp. Am J Physiol Endocrinol Metab. 2015 Feb 1;308(3):E206-E222. PubMed CrossRef
- McCarthy LC, Hosford DA, Riley JH, et al. Single-Nucleotide Polymorphism Alleles in the Insulin Receptor Gene Are Associated with Typical Migraine. Genomics. 2001 Dec;78(3):135-149. PubMed CrossRef
- Hills CE, Brunskill NJ. Cellular and physiological effects of C-peptide. Clin Sci (Lond). 2009 Apr;116(7):565-574. PubMed CrossRef
Acknowledgement
None
Disclosures
Consents: Both patients agreed to have their information published and were given a draft of the article to read prior to submission.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
