Author: Peter D. Vu * MD 1, Christy Cao BSA 2, Catherine Nguyen 3, Grant H. Chen MD, MBA 1
Author Affiliation:
1 McGovern Medical School at The University of Texas Health Science Center at Houston, 6431 Fannin, Houston, TX 77030
2 John Sealy School of Medicine at The University of Texas Medical Branch at Galveston, 301 University Blvd, Galveston, TX 77555
3 The University of Southern California, 3620 S Vermont Ave, Los Angeles, CA 90089
Competing Interests: The author/s declare no competing interests.
Issue: 10.01
DOI: 10.30756/ahmj.2023.10.01
Received: Jan 16, 2023
Revised: Mar 1, 2023
Accepted: Mar 2, 2023
Published: Mar 15, 2023
Recommended Citation: Vu PD, Cao C, Nguyen C, Chen GH. Ketamine Applications for Migraines: A Scoping Narrative Review. Ann Head Med. 2023;10:01. DOI: 10.30756/ahmj.2023.10.01
In the United States alone, nearly 40 million children and adults suffer from migraines, which are the primary cause of morbidity, quality of life reduction, and loss of productivity for persons aged 15-49. Despite their global prevalence and various available treatment options, these disabilities are often still under-treated due to the individuality of treatment regimens and effect profiles. Compelling arguments have been made for ketamine use in opioid-sparing pain management. An increase in opioid stewardship, especially during the outbreak of the novel COVID-19, has only accentuated arguments for ketamine in migraine alleviation. However, within the last 20 years, the overall body of work addressing its role has not been clearly elucidated, with variations in optimal dosage and administration routes. Thus, this review aims to consolidate previous findings of ketamine as a migraine analgesic agent and to amass the most recent burgeoning data on its effectiveness in clinical settings. A comparison of intravenous, intranasal, and subcutaneous ketamine is examined, with a discussion on pharmacology, pharmacokinetics, and results in pain outcomes analyzed.
Introduction
Headache disorders, as described by the Institute for Health Metrics and Evaluation in their 2019 Global Burden of Disease, are a leading global public health concern 1. Migraines, as its own subset category as a primary headache, ranked second among all causes of disability and debility 1, 2, affecting approximately 15% of the general population, or 1 billion people, worldwide 3, 4. In the United States alone, nearly 40 million children and adults suffer from migraines, being the primary cause of morbidity, quality of life reduction, and loss of productivity for persons aged 15-49 5.
Migraines are primary headaches manifesting as recurrent attacks of head and facial pain, sometimes accompanied by auras, or neurological disturbances 4. Current treatments range from acute and preventative medications, including NSAIDs, triptans, calcitonin gene-related peptide monoclonal antibodies, and opioids, to non-pharmacological therapies, including neuromodulators and biobehavioral therapies 3. Despite the prevalence of migraines worldwide and these treatment options, these disabilities are often still under-treated due to the individuality of treatment regimens and effect profiles 4, proposing a realm of treatments to consider adding to the therapeutic arsenal. Ketamine can potentially be one of those treatments.
Since the introduction of ketamine in the 1960s as a phencyclidine derivative, studies on its clinical utilization, mechanism of action, and psychodysleptic effects have continuously shaped its relationship within the medical community 6. Compelling arguments have been made for ketamine use in opioid-sparing pain management 7-10, procedural sedation and intubation 8, 9, 11, 12, and mood/psychiatric disorder treatments 13-17. It has also been effective in neuroprotection, seizure prophylaxis and ablation, burn pain, acute on chronic episodes of neuropathic pain, acute postoperative pain, and alcohol and substance abuse abbreviation 9. An increase in opioid stewardship, evident with a 12.8% prescription decline from 2010-2017 18, especially during the outbreak of the novel COVID-19 19, has only accentuated arguments for ketamine in migraine alleviation. However, within the last 20 years, the overall body of work addressing its role has not been clearly elucidated, with variations in optimal dosage and administration routes. Thus, this review aims to consolidate previous findings of ketamine as a migraine analgesic agent and to amass the most recent burgeoning data on its effectiveness in clinical settings.
Ketamine Pharmacology and Pharmacokinetics
As a racemic mixture of (S)- and (R)-ketamine, the chemical structure of ketamine provides an interesting dichotomy in its effects. Both enantiomers are non-competitive antagonists of the N-methyl-D-aspartate (NMDA) receptors with further interactions on μ-, k-, and σ-opioid, dopamine D2, serotonin (5-HT), cholinergic, nicotinic, monoaminergic, muscarinic, γ-aminobutyric acid (GABA), α-amino-3-hydroxyl-5-methyl-4-isoxazole propionate (AMPA), gated-ion channels, and kainite receptors 8,10, 15, 20, 21. Additional analgesic effects with ketamine metabolites and their similar structures and targeted receptors have also been reported with prolonged concentration and duration of metabolite plasma levels 22. These interactions postulate a combination of receptor interactivity resulting in neuronal cell signaling inhibition and pain transmission termination.
The same multitude of receptor interactions is hypothesized to explain the adverse side effect profile of ketamine and its retained psychomimetic and psychodysleptic properties of phencyclidine 8, 10, 15. Higher doses and administrative routes impact the severity of side effect profiles, which include dissociative anesthesia, hallucinations, feelings of dysphoria, lightheadedness, nausea, dizziness, drowsiness, ocular effects, urological deficits, neurotoxicity, and confusion 8, 9, 20. Because ketamine undergoes extensive first-pass metabolism, its oral bioavailability is relatively poor (17-29%), compared to intravenous (100%), intramuscular (93%), intranasal (45-50%), and rectal (11-25%) 8, 9, 23. Recent findings have also suggested (S)-ketamine has a 2- to 4-fold higher affinity to the NMDA receptor than its R-enantiomer counterpart, resulting in more potent effects and positing that isolation of (S)-ketamine could result in even lower doses for pain treatment 8-10, 20.
Another important pathophysiological property ketamine offers in migraine management is its potential to decrease debilitation through dampening cortical spreading depression via glutamatergic NMDA receptor inhibition 21. Cortical spreading depression is the predominant theory of auras in migraines and is described as a slow-moving propagated wave of depolarization that results in brain activity suppression 24. With up to one-third of migraines influenced by auras 25, the ability to target crippling symptoms and treat pain at the same time is efficacious.
Methods
Previous systematic reviews have evaluated ketamine use for migraines 26; newer studies and publications not included in those reviews could provide further information that could be helpful in niche patient populations. Thus, a comprehensive scoping review search was conducted with a medical research librarian instead of repeating a similar study. The goal was to find new and previously unreferenced studies to consolidate all applicable evidence to identify and analyze knowledge gaps in migraine management 27. Additionally, to stratify each study, acute migraine was defined as 0 to 14 headache days per month, chronic was 15 or more headache days per month, and status migrainosus as a headache that doesn’t respond to usual treatment or lasts longer than 72 hours 28.
The following electronic databases were searched in June 2022 and repeated in November 2022: MEDLINE via PubMed (1990 to November 2022), Web of Science (1990 to November 2022), Cochrane Library (1990 to November 2022), and EMBASE (1990 to November 2022). Search term inputs focused on “(ketamine OR esketamine OR s-ketamine) AND (migraine)” with filters limited to human trials and English-written articles. Inclusion criteria included interventional studies (randomized-control, comparative non-randomized, etc.) and observational studies (cohort, case-control, cross-sectional, case report). Randomized controlled trials were the primary focus because of their competitiveness, minimization of biases and confounding factors, and statistical reliability. Non-randomized studies were also included in our inclusion criteria because empirical evidence for the utilization of ketamine has shown benefits for patients that go beyond set benchmarks and should also be considered. Abstracts, open-label studies, commentaries, editorials, systematic literature reviews, and meta-analyses were excluded. Any references that only discussed “ketamine” or “migraine” but not both were excluded. Any references that did not discuss “ketamine” or “migraine” but mentioned other “headaches” were also excluded. Reviews and meta-analyses were manually searched for additional studies. The initial search yielded a total of 575 references. After duplicates were removed and the remainder analyzed for inclusion criteria, 14 manuscripts were included. Each study included in this review had relevant data extracted, as reported in Tables 1, 2, and 3.
Results
Routes of Administration
The subanesthetic dosing of ketamine via intranasal, subcutaneous, or intravenous routes of administration has provided a variety of dosages, adverse effects, and results.
Intravenous Ketamine
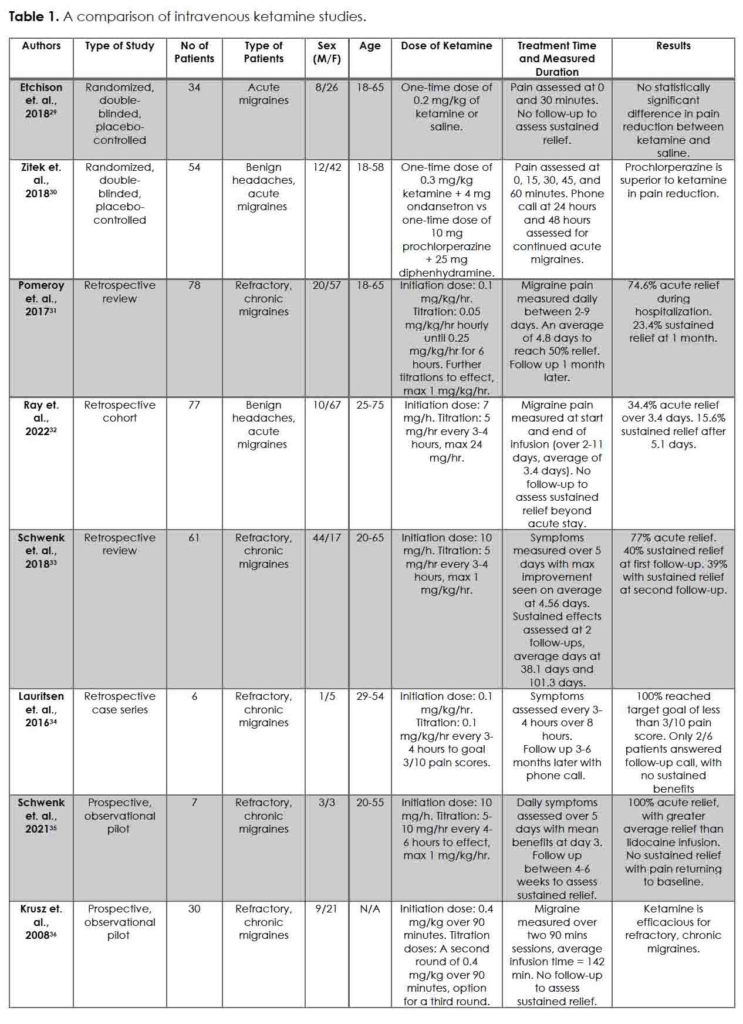
The individuality of migraine treatment between acute, preventative, and non-pharmacologic options have burgeoned, providing an array of pharmacological and non-pharmacological combinations that can be effective in alleviating pain 3. If these combinations do not, intravenous ketamine, with its concentration and utilization in an acute setting, may provide that allayment. A summary of intravenous ketamine studies referenced in this section is presented in Table 1.
Randomized controlled trials are contentious regarding the efficacy of intravenous ketamine for migraines 29, 30. Etchison et. al. conducted a randomized, double-blinded, placebo-controlled trial of 34 acute migraine subjects comparing a one-time dose of 0.2 mg/kg intravenous ketamine to an intravenous saline placebo 29. Over 30 minutes, pain reduction, functional disability scores, rescue medication request rate, and treatment satisfaction were not greater than the placebo, with both groups reporting an average reduction of pain score of 1 from baseline. However, a lack of a standardized analgesic dose of intravenous ketamine, subjective quality of pain, strict medication adherence, and population location and size limits Etchison from providing a blanket approach to intravenous ketamine for migraine management 29. Zitek et. al. concluded similar findings in their multicenter, double-blinded, randomized, controlled trial of 54 patients between intravenous prochlorperazine and 0.3 mg/kg ketamine 30. Prochlorperazine was concluded superior to ketamine in pain reduction and outcomes after an interim analysis was conducted due to concerns over ketamine-induced severe dysphoria. The dysphoria patients experienced were noticeable, however, allowing providers to voice concerns and prematurely stop the trial short of the goal of 70 patients. Additionally, providers also provided ondansetron for migraine-induced nausea, which can perpetuate further headaches. Thus, the study was underpowered, unblinded, and confounded 30.
Other intravenous, non-randomized ketamine studies have sided with the impact of ketamine on migraine treatment. Pomeroy et. al., in their retrospective review of 77 patients who failed previous aggressive outpatient and inpatient therapies, showed intravenous subanesthetic ketamine to benefit patients with refractory, chronic migraines 31. In those patients, the average length of ketamine infusion ranged from 2-9 days, with a mean of 4.8 days, at a mean rate of 0.53 mg/kg/hr. Their data showed the mean pain rating decreased by an average of 3.3 pain rating from admission to discharge (P < 0.0001). The majority of their subjects, 71.4%, had at least a 2-point improvement in pain rating at discharge, with 27.3% of their subjects maintaining this benefit within their one-month follow-up 31. Ray et. al., Schwenk et. al. (2018 and 2021), Lauritsen et. al., and Krusz et. al. report similar findings to Pomeroy, with intravenous ketamine improving migraine pain over prolonged treatment time and titrations 32-36. In some cases, sustained improvements in migraine pain were recorded (40% and 39% of patients at a follow-up visit 38 and 101 days, on average, after discharge, respectively) 33. The limitation of this collection of retrospective studies, as in any retrospective study, is the inability to generalize these findings to acute patients. Additionally, many of these studies also utilized rescue medications throughout the treatment period, making it difficult to pinpoint the individual effects of ketamine. Loss to follow-up also limits the extrapolation of sustained results or potential changes in patient habits or prophylaxis use.
Intranasal Ketamine
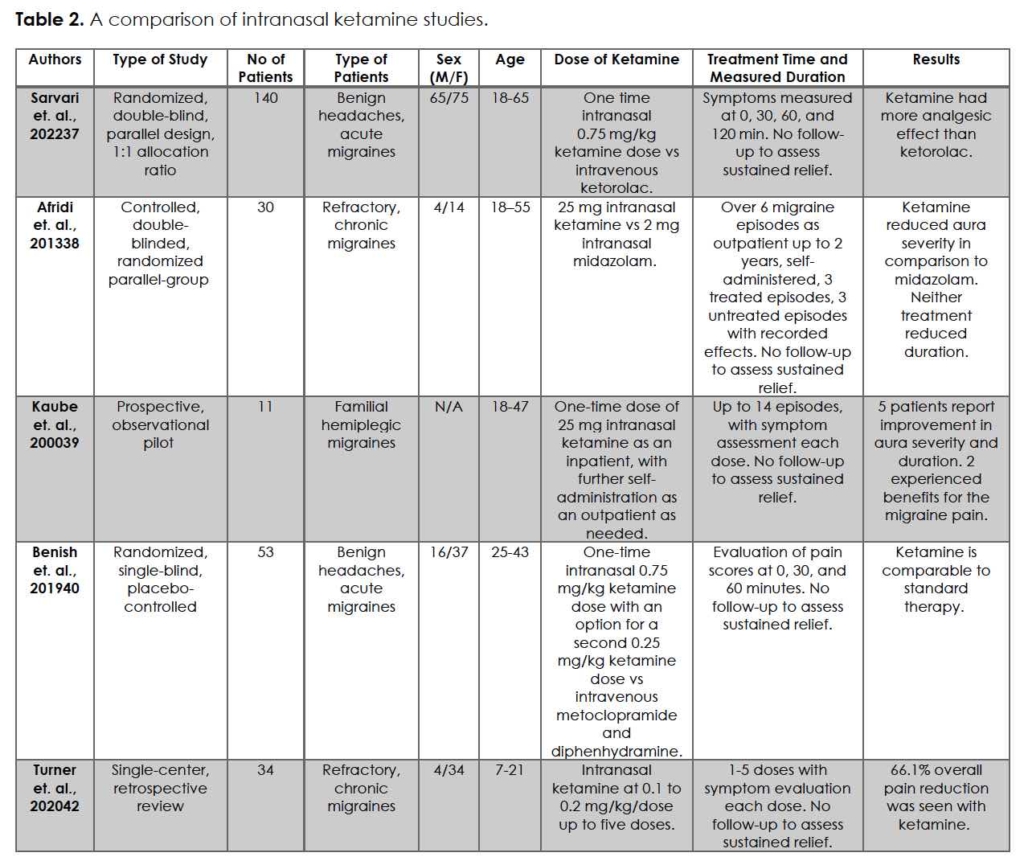
Intranasal ketamine is another option to consider for migraine and headache treatment. Its ease of access and utilization are desirable characteristics that providers consider when prescribing, especially in the acute setting. A summary of intranasal ketamine studies referenced in this section is presented in Table 2.
The three randomized-controlled trials included in this section collectively favor the use of intranasal ketamine for migraine management 37-39. Sarvari et. al. conducted a randomized, double-blinded, parallel design of 140 patients comparing a one-time intranasal 0.75 mg/kg ketamine (max 75 mg) to 30 mg intravenous ketorolac 37. Investigators were blinded to patient medications; to blind the participants, subjects in the intranasal ketamine group also received 1000 mL of normal saline intravenously while; subjects in the ketorolac group also inhaled atomized saline. The results reported an average difference in pain at 30, 60, and 120 minutes by intranasal ketamine as statistically and clinically significant (p < 0.001) compared to intravenous ketorolac. Intravenous ketorolac did provide relief for patients, hence its long-time role as a primary treatment; however, with these results, the viability of intranasal ketamine as a potential alternative option is questioned.
Afridi et. al., in their double-blinded, randomized parallel-group controlled study, demonstrated in 30 patients a reduction of migraine aura severity through 25 mg intranasal ketamine versus 2 mg intranasal midazolam 38. The patients were instructed to use their assigned nasal sprays to treat 3 migraine attacks followed by no nasal spray treatments for 3 subsequent attacks. Of those 30, 18 completed the study with 9 patients in each treatment arm. Data analysis of these 18 patients showed that neither midazolam nor ketamine reduced the duration of migraine attacks. Ketamine, however, did decrease the severity of attacks on a scaled score of 1.5 on the clinical severity scale (p = 0.032), suggesting that the baseline attack was worse than the treated attack. Kaube et. al. reports a similar potential for ketamine in migraine treatment as Afridi 39. They examined 11 patients with severe, disabling auras resulting from familial hemiplegic migraine, trialing a one-time dose of 25 mg intranasal ketamine as an inpatient, with further self-administration as-needed outpatient. Of those 11, 5 patients reported that ketamine reproducibly reduced the severity and duration of the neurologic deficits; the other 6 reported no beneficial effects.
Of the three randomized-controlled trials, Benish et. al. argued against ketamine superiority for migraines; at most, ketamine is comparable to standard therapy 40. In their randomized, single-blind, placebo-controlled trial, 53 subjects were divided between intranasal ketamine and intravenous metoclopramide and diphenhydramine solution. Initial average pain scores were 73.5 in the control versus 74.5 in the ketamine arm. The dosage of intranasal ketamine was done in two administrations: the first dose equaled 0.75 mg/kg (maximum 75 mg), and the second dose (if requested) equaled 0.25 mg/kg (maximum 25 mg). Pain scores after 30 minutes recorded an average decrease of 22.2 and 29.0 in the control and the ketamine arm, respectively. The difference between the two groups was statistically insignificant at 60 minutes. At 48-72 hours, pain and satisfaction scores were equal.
Migraine headaches are not an exclusively adult disease; pediatric migraines are a common diagnosis seen by pediatricians, emergency medicine physicians, and pediatric neurologists. According to the American Migraine Foundation, 10% of children suffer from migraines 41. Pediatric migraines are also unique in that they are more likely to present globally rather than one-sided and are usually much shorter than adult migraines 41. These clinical presentations make them harder to treat and more difficult to study. Turner et. al. retrospective review describes a cohort of 34 pediatric patients who presented to the emergency department with migraines and received serial dosing of 0.1-0.2 mg/kg/dose intranasal ketamine every 15 minutes up to 5 total doses 42. Of those 34 children, 25 (73.5%) responded with a 50% or more reduction of pain, with absolute pain scores between 0-3. Pain reduction by the first dose was seen in 9 children, with an average pain reduction of 66.1%, or an average 7.2-pain scale reduction. The pain scores differed between the ketamine responders versus non-responders: 1.4 versus 7.3, respectively (p ≤ 0.001). As discussed before, the limitations of a retrospective study exist. However, there appear to be benefits for intranasal ketamine, including no intravenous access, no necessary premedication, and expedited administration of medications over current migraine protocols.
Subcutaneous Ketamine
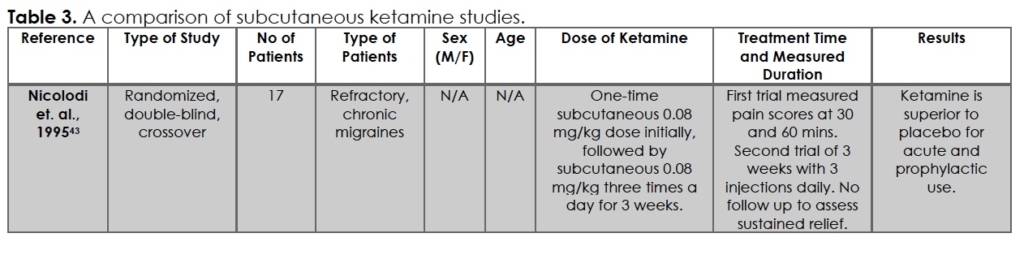
Studies on subcutaneous ketamine for migraines were limited, even to this day. An evaluation of the literature found a randomized, double-blind, cross-over study of 17 patients from 1995 by Nicolodi et. al. 43, as summarized in Table 3. Nicolodi tested acute and chronic migraine management with a one-time subcutaneous 0.08 mg/kg dose followed by subcutaneous 0.08 mg/kg three times a day for 3 weeks. This was compared to saline injections over 3 weeks by the same patients. They reported superior pain relief in acute and chronic settings seen with subcutaneous ketamine. An expert panel, convened by Orr et. al. 7, reviewed 68 RCTs through 2015 to determine the likely efficacy of injectable medications to provide first-line recommendations. They found Nicolodi to be inconclusive, concluding “No recommendation can be made regarding the role of injectable ketamine for adults who present to an emergency department with acute migraine.”
Tables
Discussion
The use of ketamine to treat pain has been a relevant discussion since its inception; studies on its use as a migraine remedy, however, are more novel. Outcomes of early studies have hinted that ketamine may alleviate migraines, but definitive conclusions with dosages, treatment times, and results are difficult to establish with the heterogeneity of each study. Of the 14 studies included, only 1 was published before 1995 and 3 before 2010. Based on our inclusion criteria, since 2010, 11 other studies have been impactful in the discussion for or against ketamine in migraines.
In the framework of intravenous ketamine, the goals of treatment for acute migraines versus refractory, chronic migraines varied significantly in the dosage and duration of treatment. Schwenk’s infusion rate of 0.76 mg/kg per hour over 5 days brought on more improvement in current and sustained pain post-discharge than the one-time doses of Etchison’s 0.2 mg/kg or Zitek’s 0.3 mg/kg emergency department management. Schwenk, Lauritsen, Ray, and Pomeroy all had infusion rates and durations greater than Zitek and Etchison 29-36, with reported improvement with ketamine. These findings for migraine treatment with ketamine for higher doses and durations mirror the guidelines for infusions for chronic pain, as written by the American Society of Regional Anesthesia and Pain Medicine in 2018 22, and the potential pharmacology and pathophysiology discussed above 8, 9. An argument on the impact of rescue medication on migraine improvement in the non-randomized studies could be made, however, Etchison and Zitek both also had rescue medications for their patients. The side effect profiles that accompany increased ketamine doses, such as dysphoria, nystagmus, confusion, and hallucination, retain plausibility in the argument against intravenous ketamine for migraine use. It is understandable why hesitancy with intravenous ketamine continues to exist, even with lower dosages or using benzodiazepines to treat adverse side effects.
The results of intranasal ketamine were more motley in nature than intravenous ketamine, with varied dosages, effects, and relief. Benish, with their 0.75 mg/kg (maximum 75 mg) first dose and 0.25 mg/kg (maximum 25 mg) second dose produced no significant difference in pain reduction compared to Afridi’s 3 doses of 25 mg, Kaube’s 1 dose of 25 mg, and Turner’s serial dosing of 0.1-0.2 mg/kg/dose (up to 5 doses) for pediatric patients 32-40, 42. Benish did report a numerical decrease in reported pain value, however. With intranasal ketamine, a discussion of bioavailability must also be considered. Compared to intravenous ketamine’s 100% plasma concentration bioavailability, intranasal ketamine’s 45-50% bioavailability could influence patient variable outcomes 8, 9, 23. The bioavailability impacts NMDA receptor inhibition and subsequent blockade of glutamatergic-induced cortical spreading depression, affecting aura and migraine pain 38, 39. NMDA receptors have also been found in the olfactory passages, with the potential for direct stimulation by intranasal ketamine 44. Thus, direct stimulation with less time for metabolite concentration and other receptor pathway effects can make intranasal ketamine more efficacious in mitigating severe migraine with less adversity.
At this time, it is difficult to discern the utility of subcutaneous ketamine. Nicolodi’s study, while a good randomized-controlled trial with suggestive efficacy for subcutaneous ketamine, is a bit outdated from 1995 43. Since then, the literature search has not provided another subcutaneous ketamine study for migraines. In addition, Orr’s panel of experts and their review of Nicolodi’s non-recommendation of subcutaneous ketamine for migraines also makes it difficult to fully support subcutaneous ketamine at this time 7. More studies, especially randomized-controlled trials for subcutaneous ketamine may be fruitful. Until then, it is all anecdotal.
In addition to the discussions on acute and chronic migraines throughout this scoping review, status migrainosus should also be addressed. As defined above, status migrainosus is a migraine that does not respond to usual treatment or lasts longer than 72 hours 28. It occurs in persons diagnosed with migraines and is typical in presentation as previous migraines. Generally, these migraines are managed with abortive migraine medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, triptans, anti-epileptics, tricyclic antidepressants (TCAs), and calcium channel blockers (CCBs) 45. Only one study found for this scoping review discussed status migrainosus, which limits a fully inclusive discussion on its management with ketamine 32. Ray et. al. reported intravenous ketamine use for status migrainosus in 10 patients, with only 2 patients having greater than 50% pain reduction and 1 patient having complete pain resolution with intravenous ketamine, providing only empirical ketamine evidence for status migrainosus. With only one study discussing status migrainosus and usual improvement with abortive medications, this limits the number of studies that could be conducted with ketamine, especially if the migraine is effectively treated without ketamine. This suggests that ketamine would only have a minor role, similar to its role in acute migraines, in status migrainosus management.
In evaluating ketamine’s efficacy for acute and chronic migraines, a discussion of other modalities and their costs for migraine treatment should also be held. While the spectrum of migraine therapeutics has significantly expanded within the past few years, it must be noted that ketamine will never be a first-line agent for migraines. Triptans, approved by the Food and Drug Administration in 1992, have been the leading option for treating acute migraines 46. Prophylactically, NSAIDs, analgesics, beta-blockers, CCBs, TCAs, antidepressants, and anti-epileptics have also been used, with newly approved pharmacological agents like anti-calcitonin gene-related peptides (anti-CGRPs) recently added to the repertoire 45. Among these treatment options, anti-CGRPs are the most expensive at $291.17 per patient per month (PPPM) in 2020 while triptans are the highest volume use at $31.92 PPPM in 2020 47. Further 2020 pricing PPPM includes $64.92 for opioids, $23.90 for NSAIDs/non-narcotic agents, $761.99 for ergotamine, $11.44 for antidepressants, $43.82 for anticonvulsants, and $13.40 for beta-blockers 47. This wide variety of pricing options and choices for patients seeking migraine medications emphasizes the heterogeneity and individuality of migraine response and the importance of expanding on all potential treatment options.
When traditional and newer pharmacologic agents fail to provide relief to patients, our discussion of ketamine for migraine therapy should be considered, particularly for refractory, chronic migraines. The cost of ketamine, while relatively cheap to manufacture, has increased upcharge between $200-$800 per infusion based on personnel, facility, training, safety protocols, insurance coverage, and equipment 48-50. While ketamine prices are on the higher end, there have been recent attempts to find ways to make ketamine more affordable to the consumer with increased discussion and awareness for ketamine indications in migraine management 51. The argument for ketamine as a cost-effective option for migraines lies in the possibility of a 1-time dose providing prolonged relief versus having monthly prescriptions. Overall costs can be decreased by utilizing higher costs medications that effectively alleviate pain and disability, reducing the need for further medications and treatments 51. Unfortunately, a limitation of these studies discussed, and other studies considered is the lack of long-term follow-up that could study the cost-benefit analysis.
Previous systematic reviews have stated that ketamine benefits are unclear, with no superiority compared to standard treatments 26. Our scoping review, which included more studies, conclude similar findings with one small caveat for chronic, refractory migraines. The studies included in our scoping review controlled for demographic differences (i.e., gender, age), which allowed for evaluations of efficacy differences in ketamine outcomes. Based on these evaluations, ketamine, particularly intravenous and intranasal ketamine, has some value for refractory, chronic migraines, especially for those who have failed multiple standard treatments 31-36, 38, 42. An argument can be made for acute migraine ketamine treatment, especially when short-term improvement can significantly improve debilitating symptoms. These cases are rare and should only be considered last when other options have been exhausted or have contraindicated side effects; of note, the use of ketamine for acute migraines has been mediocre at best 29, 43.
Limitations and Future Directions
As a scoping review, the goal of this narrative is to build upon previous articles addressing ketamine for migraine use and synthesize an overview of new evidence in relation to already published results. As discussed in the method section, a scoping review addresses different goals than a systematic review. Even then, there are limitations to a scoping review, especially when methodological limitations or bias risks of the evidence are not assessed 27. Variations in ketamine delivery between intravenous, intranasal, and subcutaneous and the differences in controls and rescue medications make the comparison of dosages and efficacy difficult. We attempted to stratify based on the delivery method to control for each group. However, it is possible that other studies have been overlooked, further biasing overall results and conclusions. Thus, this limits the ability to provide concrete guidance or policy for migraine treatment with ketamine.
The use of ketamine has current side effect limitations that preceded its effectiveness, such as but not limited to headache, dizziness, dissociation, elevated blood pressure, blurred vision, and psychiatric disorders like anxiety and hallucinations 52. One other consideration to be had, especially with migraine patients, is the concern of ketamine causing a potential increase in intracranial pressure, which has been reported since ketamine’s creation 53-55. Newer studies have been suggesting there is no correlation between intracranial pressure and ketamine use, however 53, 54, 56.
One other aspect of ketamine that has not been discussed in this review is sublingual ketamine. Sublingual ketamine is an uncomplicated way to deliver ketamine as it bypasses first-pass metabolism, allowing for higher bioavailability of active ketamine. Compared to intranasal ketamine, sublingual ketamine was comparable in active ketamine bioavailability 10, which led our group to question its viability in migraine management. Unfortunately, there is no literature on using sublingual ketamine for migraine management; future studies evaluating its effectiveness in migraine management should be considered.
Conclusion
Although ketamine is not a first-line migraine treatment, empirically, the use of ketamine can be appropriate for migraines unresponsive to standard management, especially for the management of chronic, refractory migraines. However, the level of evidence for practical use between intravenous, intranasal, and subcutaneous varies by dose range. Intranasal ketamine has the most uniform evidence for migraine treatment with all reported studies showing migraine improvement compared to intravenous or subcutaneous. However, given the small sample size of many of the studies referenced in this review and the heterogeneity of outcomes reported, definitive conclusions on ketamine administration and its use as a migraine treatment are not possible at this time. Adverse effects must be considered, especially with prolonged durations and elevated dosages, when considering the initiation of ketamine for migraines. With some benefits noted in chronic, refractory migraines with intravenous and intranasal ketamine, a future study could compare sub-dissociative intravenous ketamine versus intranasal ketamine with increasing dosages over multiple migraine attacks in a set time period via a crossover study for chronic, refractory migraine patients. A similar study could be conducted via a randomized, controlled, double-blinded trial where chronic, refractory migraine patients would be randomized into intravenous or intranasal ketamine with intravenous and intranasal normal saline as control. Regardless, larger studies are needed to establish quantifiable efficacy, enhance patient selection, clarify therapeutic dosages, arbitrate administration choice, and promote comprehension of the risks of undergoing ketamine treatment for migraines.
References
- Collaborators GBDH. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. Nov 2018;17(11):954-976. PubMed PMID: 30353868; PubMed Central PMCID: PMCPMC6191530. doi:10.1016/S1474-4422(18)30322-3
- Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z, Lifting The Burden: the Global Campaign against H. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. Dec 2 2020;21(1):137. PubMed PMID: 33267788; PubMed Central PMCID: PMCPMC7708887. doi:10.1186/s10194-020-01208-0
- Ashina M, Buse DC, Ashina H, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. Apr 17 2021;397(10283):1505-1518. PubMed PMID: 33773612. doi:10.1016/S0140-6736(20)32342-4
- Eigenbrodt AK, Ashina H, Khan S, et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol. Aug 2021;17(8):501-514. PubMed PMID: 34145431; PubMed Central PMCID: PMCPMC8321897. doi:10.1038/s41582-021-00509-5
- Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. Sep 16 2017;390(10100):1211-1259. PubMed PMID: 28919117; PubMed Central PMCID: PMCPMC5605509. doi:10.1016/S0140-6736(17)32154-2
- Rocchio RJ, Ward KE. Intranasal Ketamine for Acute Pain. Clin J Pain. Apr 1 2021;37(4):295-300. PubMed PMID: 33555694. doi:10.1097/AJP.0000000000000918
- Orr SL, Friedman BW, Christie S, et al. Management of Adults With Acute Migraine in the Emergency Department: The American Headache Society Evidence Assessment of Parenteral Pharmacotherapies. Headache. Jun 2016;56(6):911-40. PubMed PMID: 27300483. doi:10.1111/head.12835
- Zanos P, Moaddel R, Morris PJ, et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev. Jul 2018;70(3):621-660. PubMed PMID: 29945898; PubMed Central PMCID: PMCPMC6020109. doi:10.1124/pr.117.015198
- Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin Pharmacokinet. Sep 2016;55(9):1059-77. PubMed PMID: 27028535. doi:10.1007/s40262-016-0383-6
- Yang Y, Maher DP, Cohen SP. Emerging concepts on the use of ketamine for chronic pain. Expert Rev Clin Pharmacol. Feb 2020;13(2):135-146. PubMed PMID: 31990596. doi:10.1080/17512433.2020.1717947
- Merelman AH, Perlmutter MC, Strayer RJ. Alternatives to Rapid Sequence Intubation: Contemporary Airway Management with Ketamine. West J Emerg Med. May 2019;20(3):466-471. PubMed PMID: 31123547; PubMed Central PMCID: PMCPMC6526883. doi:10.5811/westjem.2019.4.42753
- Matchett G, Gasanova I, Riccio CA, et al. Etomidate versus ketamine for emergency endotracheal intubation: a randomized clinical trial. Intensive Care Med. Jan 2022;48(1):78-91. PubMed PMID: 34904190. doi:10.1007/s00134-021-06577-x
- Frohlich J, Van Horn JD. Reviewing the ketamine model for schizophrenia. J Psychopharmacol. Apr 2014;28(4):287-302. PubMed PMID: 24257811; PubMed Central PMCID: PMCPMC4133098. doi:10.1177/0269881113512909
- Wilkowska A, Szalach L, Cubala WJ. Ketamine in Bipolar Disorder: A Review. Neuropsychiatr Dis Treat. 2020;16:2707-2717. PubMed PMID: 33209026; PubMed Central PMCID: PMCPMC7670087. doi:10.2147/NDT.S282208
- Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. Apr 2018;23(4):801-811. PubMed PMID: 29532791; PubMed Central PMCID: PMCPMC5999402. doi:10.1038/mp.2017.255
- Sanacora G, Frye MA, McDonald W, et al. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry. Apr 1 2017;74(4):399-405. PubMed PMID: 28249076. doi:10.1001/jamapsychiatry.2017.0080
- Feder A, Rutter SB, Schiller D, Charney DS. The emergence of ketamine as a novel treatment for posttraumatic stress disorder. Adv Pharmacol. 2020;89:261-286. PubMed PMID: 32616209. doi:10.1016/bs.apha.2020.05.004
- Schieber LZ, Guy GP, Jr., Seth P, et al. Trends and Patterns of Geographic Variation in Opioid Prescribing Practices by State, United States, 2006-2017. JAMA Netw Open. Mar 1 2019;2(3):e190665. PubMed PMID: 30874783; PubMed Central PMCID: PMCPMC6484643. doi:10.1001/jamanetworkopen.2019.0665
- Khatri UG, Perrone J. Opioid Use Disorder and COVID-19: Crashing of the Crises. J Addict Med. Jul/Aug 2020;14(4):e6-e7. PubMed PMID: 32404651; PubMed Central PMCID: PMCPMC7236857. doi:10.1097/ADM.0000000000000684
- Jelen LA, Young AH, Stone JM. Ketamine: A tale of two enantiomers. J Psychopharmacol. Feb 2021;35(2):109-123. PubMed PMID: 33155503; PubMed Central PMCID: PMCPMC7859674. doi:10.1177/0269881120959644
- Hoffmann J, Charles A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurotherapeutics. Apr 2018;15(2):361-370. PubMed PMID: 29508147; PubMed Central PMCID: PMCPMC5935645. doi:10.1007/s13311-018-0616-5
- Cohen SP, Bhatia A, Buvanendran A, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. Jul 2018;43(5):521-546. PubMed PMID: 29870458; PubMed Central PMCID: PMCPMC6023575. doi:10.1097/AAP.0000000000000808
- Li L, Vlisides PE. Ketamine: 50 Years of Modulating the Mind. Front Hum Neurosci. 2016;10:612. PubMed PMID: 27965560; PubMed Central PMCID: PMCPMC5126726. doi:10.3389/fnhum.2016.00612
- Telles JPM, Welling LC, Coelho A, Rabelo NN, Teixeira MJ, Figueiredo EG. Cortical spreading depolarization and ketamine: a short systematic review. Neurophysiol Clin. Mar 2021;51(2):145-151. PubMed PMID: 33610431. doi:10.1016/j.neucli.2021.01.004
- Dodick DW. A Phase-by-Phase Review of Migraine Pathophysiology. Headache. May 2018;58 Suppl 1:4-16. PubMed PMID: 29697154. doi:10.1111/head.13300
- Chah N, Jones M, Milord S, Al-Eryani K, Enciso R. Efficacy of ketamine in the treatment of migraines and other unspecified primary headache disorders compared to placebo and other interventions: a systematic review. J Dent Anesth Pain Med. Oct 2021;21(5):413-429. PubMed PMID: 34703891; PubMed Central PMCID: PMCPMC8520840. doi:10.17245/jdapm.2021.21.5.413
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. Nov 19 2018;18(1):143. PubMed PMID: 30453902; PubMed Central PMCID: PMCPMC6245623. doi:10.1186/s12874-018-0611-x
- Katsarava Z, Buse DC, Manack AN, Lipton RB. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. Feb 2012;16(1):86-92. PubMed PMID: 22083262; PubMed Central PMCID: PMCPMC3258393. doi:10.1007/s11916-011-0233-z
- Etchison AR, Bos L, Ray M, et al. Low-dose Ketamine Does Not Improve Migraine in the Emergency Department: A Randomized Placebo-controlled Trial. West J Emerg Med. Nov 2018;19(6):952-960. PubMed PMID: 30429927; PubMed Central PMCID: PMCPMC6225951. doi:10.5811/westjem.2018.8.37875
- Zitek T, Gates M, Pitotti C, et al. A Comparison of Headache Treatment in the Emergency Department: Prochlorperazine Versus Ketamine. Ann Emerg Med. Mar 2018;71(3):369-377 e1. PubMed PMID: 29033296. doi:10.1016/j.annemergmed.2017.08.063
- Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine Infusions for Treatment Refractory Headache. Headache. Feb 2017;57(2):276-282. PubMed PMID: 28025837. doi:10.1111/head.13013
- Ray JC, Cheng S, Tsan K, et al. Intravenous Lidocaine and Ketamine Infusions for Headache Disorders: A Retrospective Cohort Study. Front Neurol. 2022;13:842082. PubMed PMID: 35356451; PubMed Central PMCID: PMCPMC8959588. doi:10.3389/fneur.2022.842082
- Schwenk ES, Dayan AC, Rangavajjula A, et al. Ketamine for Refractory Headache: A Retrospective Analysis. Reg Anesth Pain Med. Nov 2018;43(8):875-879. PubMed PMID: 29923953. doi:10.1097/AAP.0000000000000827
- Lauritsen C, Mazuera S, Lipton RB, Ashina S. Intravenous ketamine for subacute treatment of refractory chronic migraine: a case series. J Headache Pain. Dec 2016;17(1):106. PubMed PMID: 27878523; PubMed Central PMCID: PMCPMC5120050. doi:10.1186/s10194-016-0700-3
- Schwenk ES, Torjman MC, Moaddel R, et al. Ketamine for Refractory Chronic Migraine: An Observational Pilot Study and Metabolite Analysis. J Clin Pharmacol. Nov 2021;61(11):1421-1429. PubMed PMID: 34125442; PubMed Central PMCID: PMCPMC8769496. doi:10.1002/jcph.1920
- Krusz J CJ, Hall S. Efficacy of IV ketamine in treating refractory migraines in the clinic. The Journal of Pain. 2008;9(S2)(4):30. doi:10.1016/j.jpain.2008.01.139
- Sarvari HR, Baigrezaii H, Nazarianpirdosti M, Meysami A, Safari-Faramani R. Comparison of the efficacy of intranasal ketamine versus intravenous ketorolac on acute non-traumatic headaches: a randomized double-blind clinical trial. Head Face Med. Jan 3 2022;18(1):1. PubMed PMID: 34980184; PubMed Central PMCID: PMCPMC8722273. doi:10.1186/s13005-021-00303-0
- Afridi SK, Giffin NJ, Kaube H, Goadsby PJ. A randomized controlled trial of intranasal ketamine in migraine with prolonged aura. Neurology. Feb 12 2013;80(7):642-7. PubMed PMID: 23365053. doi:10.1212/WNL.0b013e3182824e66
- Kaube H, Herzog J, Kaufer T, Dichgans M, Diener HC. Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology. Jul 12 2000;55(1):139-41. PubMed PMID: 10891926. doi:10.1212/wnl.55.1.139
- Benish T, Villalobos D, Love S, et al. The THINK (Treatment of Headache with Intranasal Ketamine) Trial: A Randomized Controlled Trial Comparing Intranasal Ketamine with Intravenous Metoclopramide. J Emerg Med. Mar 2019;56(3):248-257 e1. PubMed PMID: 30910061. doi:10.1016/j.jemermed.2018.12.007
- Dennis A, Matthew LJ, Yan Z, Holger C. Predictors of Headache/Migraine and the Use of Complementary Medicine in U.S. Children: A Population-Based Analysis of 2017 National Health Interview Survey Data. J Integr Complement Med. Jan 2022;28(1):60-66. PubMed PMID: 35085015. doi:10.1089/jicm.2021.0117
- Turner AL, Shandley S, Miller E, Perry MS, Ryals B. Intranasal Ketamine for Abortive Migraine Therapy in Pediatric Patients: A Single-Center Review. Pediatr Neurol. Mar 2020;104:46-53. PubMed PMID: 31902550. doi:10.1016/j.pediatrneurol.2019.10.007
- Nicolodi M, Sicuteri F. Exploration of NMDA receptors in migraine: therapeutic and theoretic implications. Int J Clin Pharmacol Res. 1995;15(5-6):181-9. PubMed PMID: 8835616.
- McCulloch PF, DiNovo KM, Westerhaus DJ, et al. Trigeminal Medullary Dorsal Horn Neurons Activated by Nasal Stimulation Coexpress AMPA, NMDA, and NK1 Receptors. ISRN Neurosci. 2013;2013:152567. PubMed PMID: 24967301; PubMed Central PMCID: PMCPMC4045565. doi:10.1155/2013/152567
- Ailani J, Burch RC, Robbins MS, Board of Directors of the American Headache S. The American Headache Society Consensus Statement: Update on integrating new migraine treatments into clinical practice. Headache. Jul 2021;61(7):1021-1039. PubMed PMID: 34160823. doi:10.1111/head.14153
- Yang CP, Liang CS, Chang CM, et al. Comparison of New Pharmacologic Agents With Triptans for Treatment of Migraine: A Systematic Review and Meta-analysis. JAMA Netw Open. Oct 1 2021;4(10):e2128544. PubMed PMID: 34633423; PubMed Central PMCID: PMCPMC8506232. doi:10.1001/jamanetworkopen.2021.28544
- Nguyen JL, Munshi K, Peasah SK, et al. Trends in utilization and costs of migraine medications, 2017-2020. J Headache Pain. Aug 28 2022;23(1):111. PubMed PMID: 36031609; PubMed Central PMCID: PMCPMC9420279. doi:10.1186/s10194-022-01476-y
- Ramos M BW, Alpert M. The New Ketamine-Based Antidepressant Is a Rip-Off. Vice. Accessed March, 2023. https://www.vice.com/en/article/pajkjy/opinion-the-new-ketamine-based-antidepressant-is-a-rip-off
- Samuel T. Wilkinson M. What Is the Deal With Esketamine? 2020;37(4). https://www.psychiatrictimes.com/view/what-deal-esketamine
- Rapoport AM, Adelman JU. Cost of migraine management: a pharmacoeconomic overview. Am J Manag Care. Apr 1998;4(4):531-45. PubMed PMID: 10179912.
- Marianna Vinokur DFC, MD; Patrick Sullivan, DO; and Michael J. Marmura, MD. Ketamine for Intractable Headache. Online Article. Practical Neurology. 2022;Nov-Dec; 21(1):9. https://practicalneurology.com/articles/2022-nov-dec/ketamine-for-intractable-headache/pdf
- Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. Jan 2018;5(1):65-78. PubMed PMID: 28757132. doi:10.1016/S2215-0366(17)30272-9
- Cohen L, Athaide V, Wickham ME, Doyle-Waters MM, Rose NG, Hohl CM. The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: a systematic review. Ann Emerg Med. Jan 2015;65(1):43-51 e2. PubMed PMID: 25064742. doi:10.1016/j.annemergmed.2014.06.018
- Loflin R, Koyfman A. When used for sedation, does ketamine increase intracranial pressure more than fentanyl or sufentanil? Ann Emerg Med. Jan 2015;65(1):55-6. PubMed PMID: 25233812. doi:10.1016/j.annemergmed.2014.08.017
- Green SM, Andolfatto G, Krauss BS. Ketamine and intracranial pressure: no contraindication except hydrocephalus. Ann Emerg Med. Jan 2015;65(1):52-4. PubMed PMID: 25245275. doi:10.1016/j.annemergmed.2014.08.025
- Zeiler FA, Teitelbaum J, West M, Gillman LM. The ketamine effect on intracranial pressure in nontraumatic neurological illness. J Crit Care. Dec 2014;29(6):1096-106. PubMed PMID: 24996763. doi:10.1016/j.jcrc.2014.05.024
Declarations/Disclosures
Consent/Permission/Ethics Approval: Not applicable.
Funding/Conflicts of interest: In compliance with the ICMJE uniform disclosure form, author declares the following:
Payment/services info: Author has declared that no financial support was received from any organization for the submitted work.
Financial relationships: Author have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: Author have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Authors’ contributions:
PDV conducted a literature review, wrote the manuscript, and edited it with the help of CC and CN with assistance and direction from GHC.
CC assisted with the literature review, writing the manuscript, and editing with PDV and CN with assistance and direction from GHC.
CN assisted with the literature review, writing the manuscript, and editing with PDV and CC with assistance and direction from GHC.
GHC conceived the presented idea. Provided discussion and edits for the manuscript. Provided supervision with writing.
