Author: Marzieh Hassanijirdehi, MD-MPH 1, Amir Soheil Tolebeyan*, MD 1
Author Affiliation:
1 Department of Neurology, Tufts Medical Center, Boston, MA
Competing Interests: The author/s declare no competing interests.
Issue: 12.03
DOI: 10.30756/ahmj.2024.12.03
Received: Sept 30, 2024
Accepted: Nov 19, 2024
Published: Dec 13, 2024
Recommended Citation: Hassanijirdehi M, Tolebeyan AS. New Daily Persistent Headaches with Migrainous Features Following Proton Pump Inhibitor Use: A Case Report and Literature Review. Ann Head Med. 2024;12:03. DOI: 10.30756/ahmj.2024.12.03
Migraine disease is a prevalent neurological disorder characterized by recurrent pulsating unilateral headaches, occasionally accompanied by sensory disturbances. Comorbidities such as cardiovascular complications, epilepsy, anxiety, depression, and sleep disorders are commonly associated with migraines. Proton pump inhibitors (PPIs) are commonly prescribed for acid-related conditions, but their use has been linked to adverse effects, including headaches. This case report presents the first case of new daily persistent headaches and chronic migraine disease associated with using proton pump inhibitors.
Introduction
Migraine disease is a commonly occurring neurological disorder distinguished by recurrent episodes of pulsating unilateral headaches that can range from moderate to severe in intensity. Around 33% of individuals with migraines encounter an aura characterized by transient neurological manifestations such as disturbances in vision, sensation, speech, brainstem, and motor functions 1. Migraine disease has been clinically associated with an elevated likelihood of comorbidities, such as heightened incidences of cardiovascular and cerebrovascular complications (e.g., stroke, dyslipidemia, and hypertension), epilepsy, anxiety, depression, sleep disorders, endocrine disorders, and other pain-related and medication-related conditions 2-4. Identifying and mitigating modifiable risk factors for migraine headaches is essential to prevent many troublesome outcomes, given the considerable impact on disability, quality of life, and economic burden associated with chronic migraine treatment 2, 5. Established risk factors associated with migraine headaches comprise younger age, female gender, alcohol, cigarette smoking, obesity, low economic status, lower levels of education, depressed mood, stressful lifestyle, poor nutrition, and specific medications 1, 5.
Proton pump inhibitors (PPIs) are frequently prescribed to treat acid-related disorders such as peptic ulcers and gastroesophageal reflux disease. The utilization of PPIs has been linked to unfavorable side effects in 1-4% of patients, such as headache, nausea, diarrhea, abdominal pain, constipation, dizziness, fatigue, rash, and pruritus 6. Proton Pump Inhibitors (PPIs) tolerability has been extensively examined in randomized clinical trials. The most frequently reported adverse event is headache, with a prevalence ranging from 2.9% to 6.9% among omeprazole users, 1.3% among pantoprazole users, 2.4% to 6.0% among rabeprazole users, and 3.8% to 8.8% among lansoprazole users 6, 7. In a study conducted to investigate the features of PPI-related headaches and their predisposing factors, it was revealed that women, patients with previous analgesic use, and patients reporting other adverse events were at a higher risk of developing headaches. Tension-type headaches accounted for about two-thirds of PPI-related cases, while migraine headaches constituted approximately one-third 8.
In contrast to individuals from different ethnic backgrounds, East Asians have a slower metabolic rate of PPIs due to a genetically regulated decrease in the hepatic cytochrome p450 enzyme activity, which plays a vital role in the breakdown of PPIs 9. A retrospective nested case-control study in Korea, conducted to determine the correlation between preceding PPI medication and new onset migraine headaches with or without aura, showed that prior PPI use might increase the probability of migraine headaches either with or without aura, which was independent of the history and the duration of PPI use, and was still significant even after adjusting for possible confounders of migraine headaches 2. In addition, two other studies performed in the United States and the United Kingdom have demonstrated a specific relation between migraine headaches and PPI use 10, 11.
To our best knowledge, no study has documented the emergence of new daily persistent headaches (NDPH) with migrainous features in a patient after the administration of a PPI. In the forthcoming case report, we aim to elucidate the manifestation of a patient with new daily persistent headaches with migrainous features after the initiation of proton pump inhibitor (PPI) therapy.
Methodology
We conducted a search in PubMed using the keywords PPI, Proton Pump Inhibitors, Chronic Migraine, NDPH, PPIs and Migraine, as well as PPIs and Headaches without any date or language restrictions. Two reviewers completed the search. We reviewed studies that provided background information on the relationship between PPIs and headaches.
Case Presentation
A 23-year-old male with a history of GERD presented with daily headaches that began a few days following starting omeprazole for gastroesophageal reflux. The headaches were characterized as sharp, with a severity rating of 8/10, primarily affecting the temporal and frontal regions, occasionally extending to the occipital area and radiating to both eyes. The episodes lasted a few hours, resolved spontaneously, and recurred throughout the day. The patient reported photophobia, mild phonophobia, and fatigue as accompanying symptoms. Notably, he denied dizziness and nausea.
Additionally, the patient experienced continuous bilateral non-pulsating tinnitus that started a few months after the onset of his headaches. He did not have a confirmed diagnosis or history of migraine disease prior to taking the PPI. The neurological examination of the patient revealed no abnormal findings. Cranial nerves, motor function, coordination, sensory perception, and reflexes were all within normal limits. The patient exhibited no signs of neurological deficits or abnormalities.
The patient was diagnosed with new daily persistent headaches with migrainous features. Topiramate was initiated. However, the patient discontinued the treatment due to a lack of efficacy.
Suspecting cervicogenic headaches, Tizanidine 4 mg was prescribed, but the patient did not try it. Tinnitus prompted a referral to an ear, nose, and throat (ENT) specialist, who conducted an audiogram that yielded normal results. Cerumen obstructing the right ear canal was identified and removed, but the tinnitus persisted. The patient also reported experiencing lightheadedness upon standing and intermittent chest pain, which prompted an evaluation in the emergency department. Normal results were obtained from an electrocardiogram (EKG), chest X-ray, and bedside echo. A head computed tomography (CT) scan was also normal.
Ultimately, nortriptyline was added for prophylaxis, and sumatriptan as an abortive therapy. However, the patient only tried sumatriptan once, which worked well but caused nausea. He never tried nortriptyline. At the 6-week follow-up visit, the patient reported a remarkable 95% reduction in headache frequency and intensity a few days after discontinuing omeprazole. The patient reported only a couple of mild (2/10) pain a month during the follow-up with the headache specialist about two months after he stopped omeprazole.
Discussion
According to the most recent iteration of the International Classification of Headache attributes, NDPH is an infrequent primary headache, defined as an enduring headache following a distinct temporal pattern, commencing abruptly and continuing daily without relief. NDPH mainly impacts individuals who have not previously experienced headaches. Its significance lies in its unrelenting nature and resistance to treatment. This condition frequently leads to disabling effects, markedly impacting an individual’s quality of life and potentially resulting in psychiatric disorders. NDPH may manifest analogous qualities to tension-type headaches or migrainous episodes, with or without accompanying symptomatic features such as photophobia or emesis 12. Chronic migraines can be debilitating and significantly impact a patient’s quality of life. Identifying the underlying cause of chronic migraines is crucial for effective management. In this case report, we presented a patient with a history of gastroesophageal reflux disease (GERD) who initially sought headache management. The patient experienced daily headaches accompanied by photophobia, mild phonophobia, fatigue, and bilateral non-pulsating tinnitus. Despite various treatment attempts, including topiramate and tizanidine, the patient found no relief. However, upon discontinuation of omeprazole, the patient reported a remarkable reduction in headache frequency and intensity, indicating a potential association between PPI usage and chronic migraines/NDPH.
The temporal relationship between the initiation of omeprazole and the onset of daily headaches suggests a possible cause-effect relationship. While PPIs are generally considered safe and well-tolerated, they have been associated with several adverse effects, including headaches. Although PPI-induced headache is not widely investigated, previous studies have shown that patients without any background of migraine headaches are more susceptible to developing migraine-like headaches after PPI initiation 10, 11 (Table 1).
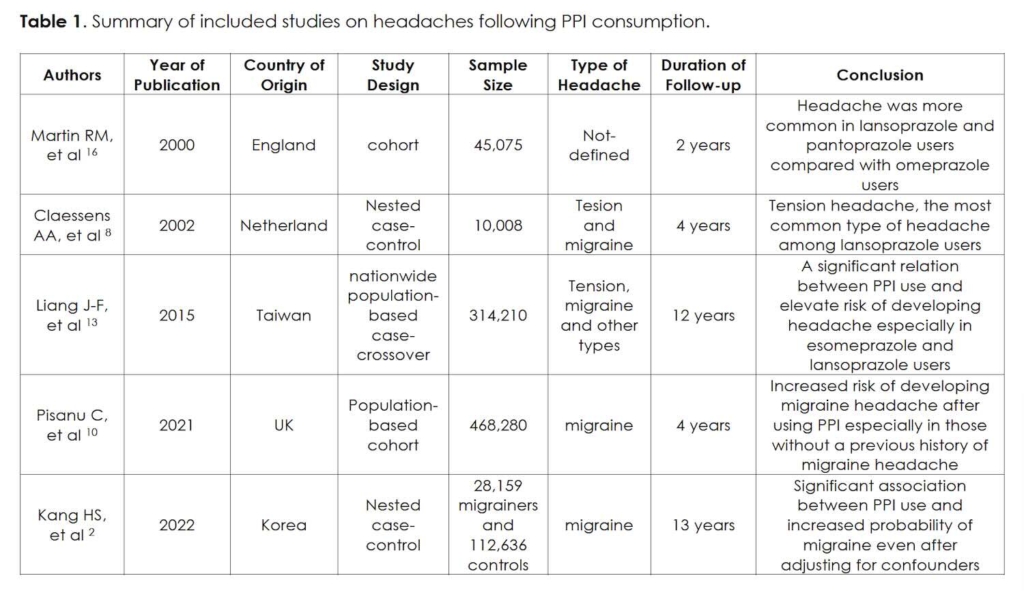
In a case-control study in Korea, it was proposed that there was an association between the duration of PPI consumption and the development of migraine-like headaches, indicating that those who used PPI for more than 30 days were more likely to experience migraine headaches 2. Contrarily, another study conducted in Taiwan demonstrated that the risk of migraine headaches was not significantly increased in PPI users 13. Furthermore, the theory of the gut-brain axis recommends an association between migraines and gastrointestinal problems, which might be mediated through various factors, including inflammatory mediators, GI microbial flora, neuropeptides, neurotransmitters, stress hormones, and different nutrients 14. Kurth et al. have suggested that the headache that occurred following the initiation of PPI could be confounded by an underlying GI condition that leads to a misconception 15.
It is of significance to highlight that our patient was a male of Asian descent. This is noteworthy due to findings from prior research, which have demonstrated that, in contrast to individuals from different ethnic backgrounds, East Asians exhibit a decreased metabolic rate of proton pump inhibitors (PPIs). This can be attributed to a genetically attenuated expression of hepatic cytochrome p450 enzyme, a pivotal factor in the metabolism of PPIs 2.
PPIs effectively hinder acid secretion by irreversibly inhibiting luminal H+/K+-ATPases in gastric parietal cells, resulting in varying electrolyte imbalances. Many PPIs traverse the blood-brain barrier, raising the prospect of direct cerebral influence. Central nervous system implications of H+/K+-ATPase, encompassing proton gradient modulation, neurotransmitter processes, and pH modulation through vesicular adenosine triphosphatase, are implicated, especially in conditions lowering brain PH. Clinical and experimental data highlight ionic perturbations during migraines. Vesicular proton pump inhibition could elevate cerebrospinal fluid K+ levels, potentially triggering migraines with aura or raising Na+ levels, amplifying neuronal excitability. Cytochrome P450 2C19 (CYP2C19) may also play a role, with genetic variations influencing PPI metabolism and potentially modulating migraines. Variability in CYP2C19 phenotypes, such as poor-to-intermediate metabolizers and rapid/ultra-rapid metabolizers, links patient-specific pharmacokinetics and pharmacogenomics to PPI-induced migraines 2. Still, the precise mechanism regarding the association between PPIs and headaches remains ambiguous.
In the context of a case-control study conducted by Kang et al., involving 28,159 patients diagnosed with migraines and 112,636 matched control individuals, the research revealed a notable link between prior usage of PPIs and an increased susceptibility to migraines, whether accompanied by aura or not. Remarkably, this association persisted regardless of the temporal aspect (past or present) and the duration of PPI use, even after meticulous adjustment for a comprehensive array of potential confounding variables pertaining to migraine headaches 2. In an investigation conducted by Claessens et al., leveraging data from an extensive prospective follow-up study involving 10,008 individuals using lansoprazole in routine clinical practice, the study revealed an incidence density of 7.2 headaches per 1000 person-years of lansoprazole usage. Notably, the proportion of patients reporting headaches was relatively modest, at 2.5%, as opposed to the frequencies observed in clinical trials involving lansoprazole, which ranged from 3.8% to 8.8%. This article also explored various characteristics of headaches and showed tension-type headaches as the most prevalent headache type among lansoprazole users 8.
In a nationwide case-crossover study involving the population of Taiwan conducted by Liang et al., it was highlighted that the utilization of PPIs correlated with a temporary surge in the risk of developing headaches. The overall likelihood of experiencing headaches showed an increase of up to 1.41 times within a 7-day timeframe following PPI usage. Notably, the extent of this headache risk variation was found to be dependent on the specific type of PPI employed, with esomeprazole and lansoprazole exhibiting a heightened risk level comparable to that associated with nitrates. Moreover, the study indicated that female patients manifested a greater vulnerability to headaches linked to PPI consumption 13.
In an English cohort study by Martin et al., a prominent observation emerged: the most frequently reported adverse events associated with omeprazole, lansoprazole, and pantoprazole were relatively uncommon. Specifically, the incidence rates of headaches among these groups were found to be 0.10, 0.17, and 0.15 per 1000 days of exposure, respectively 16.
In an investigation led by Pisanu et al., utilizing data from the extensive UK Biobank—a substantial population-based cohort comprising over 500,000 individuals aged between 40 and 69 from the UK—an elevated prevalence of migraines was noted among participants who received PPI treatment. Moreover, among participants initially without a migraine diagnosis, PPI usage was linked to a heightened occurrence of probable Migraine Without Aura and Migraine with Aura during the follow-up period 10.
It is worth noting that our patient’s presentation initially raised suspicion of new daily persistent headaches with migrainous features. However, the lack of response to standard migraine treatment and the subsequent improvement upon discontinuation of omeprazole suggest that PPI-induced chronic migraines were the primary etiology in this case. The presence of associated symptoms, including photophobia, mild phonophobia, and fatigue, further supports the diagnosis of migraine disease.
The resolution of the patient’s tinnitus following cessation of omeprazole is an intriguing finding. Tinnitus is a complex condition with multiple etiologies, including otological, neurological, and medication-induced causes. While the exact mechanism underlying PPI-induced tinnitus remains unclear, it is possible that the cessation of omeprazole resolved the underlying mechanism responsible for the patient’s tinnitus.
Conclusion
This case report highlights the potential association between PPI usage, omeprazole, and the development of chronic migraine-like headaches. Healthcare providers should be aware of this possible adverse effect and consider it in patients presenting with new-onset migraines. Timely recognition and appropriate management, including discontinuing PPIs, are crucial for improving patient outcomes. Further research, including prospective studies and mechanistic investigations, is warranted to establish a definitive causal relationship between PPIs and chronic migraines and provide evidence-based recommendations for management.
References
- Oie LR, Kurth T, Gulati S, Dodick DW. Migraine and risk of stroke. J Neurol Neurosurg Psychiatry. Jun 2020;91(6):593-604. PubMed PMID: 32217787; PubMed Central PMCID: PMCPMC7279194. doi:10.1136/jnnp-2018-318254
- Kang HS, Kim SY, Kim JH, et al. Association between Migraines and Prior Proton Pump Inhibitor Use: A Nested Case-Control Study Using a National Health Screening Cohort. Pharmaceuticals (Basel). Nov 10 2022;15(11)PubMed PMID: 36355557; PubMed Central PMCID: PMCPMC9694889. doi:10.3390/ph15111385
- Brunelli N, Altamura C, Mallio CA, et al. Cerebral Hemodynamics, Right-to-Left Shunt and White Matter Hyperintensities in Patients with Migraine with Aura, Young Stroke Patients and Controls. Int J Environ Res Public Health. Jul 14 2022;19(14)PubMed PMID: 35886428; PubMed Central PMCID: PMCPMC9318654. doi:10.3390/ijerph19148575
- Kim Y PS, Kim E, Je NK. Utilization of Preventive Therapy in Korean Migraine Patients. Korean J Clin Pharm. 2021;31(1):35-43. doi:10.24304/kjcp.2021.31.1.35
- Burch RC, Buse DC, Lipton RB. Migraine: Epidemiology, Burden, and Comorbidity. Neurol Clin. Nov 2019;37(4):631-649. PubMed PMID: 31563224. doi:10.1016/j.ncl.2019.06.001
- Langtry HD, Markham A. Rabeprazole: a review of its use in acid-related gastrointestinal disorders. Drugs. Oct 1999;58(4):725-42. PubMed PMID: 10551440. doi:10.2165/00003495-199958040-00014
- Langtry HD, Wilde MI. Lansoprazole. An update of its pharmacological properties and clinical efficacy in the management of acid-related disorders. Drugs. Sep 1997;54(3):473-500. PubMed PMID: 9279507. doi:10.2165/00003495-199754030-00010
- Claessens AA, Heerdink ER, van Eijk JT, Lamers CB, Leufkens HG. Determinants of headache in lansoprazole users in The Netherlands: results from a nested case-control study. Drug Saf. 2002;25(4):287-95. PubMed PMID: 11994030. doi:10.2165/00002018-200225040-00005
- Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. Oct 2008;64(10):935-51. PubMed PMID: 18679668. doi:10.1007/s00228-008-0538-y
- Pisanu C, Welander NZ, Rukh G, Schioth HB, Mwinyi J. Association between migraine prevalence, treatment with proton-pump inhibitors and CYP2C19 phenotypes in UK Biobank. Biomed Pharmacother. Nov 2021;143:112234. PubMed PMID: 34649359. doi:10.1016/j.biopha.2021.112234
- Makunts T, Alpatty S, Lee KC, Atayee RS, Abagyan R. Proton-pump inhibitor use is associated with a broad spectrum of neurological adverse events including impaired hearing, vision, and memory. Sci Rep. Nov 21 2019;9(1):17280. PubMed PMID: 31754136; PubMed Central PMCID: PMCPMC6872761. doi:10.1038/s41598-019-53622-3
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. Jan 2018;38(1):1-211. PubMed PMID: 29368949. doi:10.1177/0333102417738202
- Liang JF, Chen YT, Fuh JL, et al. Proton pump inhibitor-related headaches: a nationwide population-based case-crossover study in Taiwan. Cephalalgia. Mar 2015;35(3):203-10. PubMed PMID: 24853165. doi:10.1177/0333102414535114
- Arzani M, Jahromi SR, Ghorbani Z, et al. Gut-brain Axis and migraine headache: a comprehensive review. J Headache Pain. Feb 13 2020;21(1):15. PubMed PMID: 32054443; PubMed Central PMCID: PMCPMC7020496. doi:10.1186/s10194-020-1078-9
- Kurth T. Is the way to headache through the stomach? Cephalalgia. Mar 2015;35(3):201-2. PubMed PMID: 24847168. doi:10.1177/0333102414535112
- Martin RM, Dunn NR, Freemantle S, Shakir S. The rates of common adverse events reported during treatment with proton pump inhibitors used in general practice in England: cohort studies. Br J Clin Pharmacol. Oct 2000;50(4):366-72. PubMed PMID: 11012560; PubMed Central PMCID: PMCPMC2014999. doi:10.1046/j.1365-2125.2000.00262.x
Declarations/Disclosures
Consent/Permission/Ethics Approval: Informed consent was obtained from the patient for the publication of this case report.
Funding/Conflicts of interest: In compliance with the ICMJE uniform disclosure form, author declares the following:
Payment/services info: Author has declared that no financial support was received from any organization for the submitted work.
Financial relationships: Author have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: Author have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
