Author: Samer Narouze MD PhD * 1, Alexander Feoktistov MD PhD 2
Author Affiliation:
1 Professor and Chairman, Center for Pain Medicine, Western Reserve Hospital, Cuyahoga Falls, OH
2 Diamond Headache Clinic, Chicago, IL, USA
Research Support: Support was provided solely from institutional and/or departmental sources.
Competing Interests: The author/s declare no competing interests.
Issue: 02.06
DOI: doi.org/10.30756/ahmj.2020.02.06
Received: April 22, 2020
Revised: April 22, 2020
Accepted: April 23, 2020
Published: April 28, 2020
Recommended Citation: Narouze S, Feoktistov A. OnabotulinumtoxinA Injections for Patients with Chronic Migraine During the COVID-19 Pandemic. Ann Head Med. 2020;02:06. DOI: 10.30756/ahmj.2020.02.06
Headaches are among the top prevalent conditions that physicians encounter in their daily practice. Migraine headaches affect nearly15% of the United States population (approximately 45 million).1,2 Over 50% of all patients with migraine report significant or severe impairment and disability.2 Migraine is the leading cause of years lived with disability among patients between ages 15 and 49 years old – the most productive years.3
According to the World Health Organization headache report, up to 4% of the world’s population experience chronic migraine (headaches occurring on at least 15 days per month with at least 8 of these headaches meeting migraine criteria).4
Approximately, 65% of patients with episodic migraine and 75% of patients with chronic migraine have missed family events and activities in the past month due to migraine-related impairment.5 Migraine also significantly impacts work-related activities. It has been estimated that 11% of patients with chronic migraine have been missing at least 1 day of work per week in the past 2 weeks.6 Based on migraine attack frequency and related disability, headache experts suggest offering prophylactic treatment options to patients with four migraine attacks per month or more. 7 Nevertheless, it has been estimated that only 26-29% of patients continue to adhere to their prophylactic treatment regimen at 6 months and only 17-20% continue to use their oral preventive medications at 12 months. 8,9 OnabotulinumtoxinA injection is an established FDA-approved therapy for chronic migraine prevention.
Headaches are among the top prevalent conditions that physicians encounter in their daily practice. Migraine headaches affect nearly15% of the United States population (approximately 45 million).1,2 Over 50% of all patients with migraine report significant or severe impairment and disability.2 Migraine is the leading cause of years lived with disability among patients between ages 15 and 49 years old – the most productive years.3
According to the World Health Organization headache report, up to 4% of the world’s population experience chronic migraine (headaches occurring on at least 15 days per month with at least 8 of these headaches meeting migraine criteria).4
Approximately, 65% of patients with episodic migraine and 75% of patients with chronic migraine have missed family events and activities in the past month due to migraine-related impairment.5 Migraine also significantly impacts work-related activities. It has been estimated that 11% of patients with chronic migraine have been missing at least 1 day of work per week in the past 2 weeks.6 Based on migraine attack frequency and related disability, headache experts suggest offering prophylactic treatment options to patients with four migraine attacks per month or more. 7 Nevertheless, it has been estimated that only 26-29% of patients continue to adhere to their prophylactic treatment regimen at 6 months and only 17-20% continue to use their oral preventive medications at 12 months. 8,9 OnabotulinumtoxinA injection is an established FDA-approved therapy for chronic migraine prevention.
The COVID Pandemic and OnabotulinumtoxinA Injection
Novel Coronavirus-2019 (COVID-19) infection can cause severe acute respiratory syndrome (SARS-CoV-2). Older patients, as well as patients with significant comorbidities (e.g. immunosuppression, cardiovascular disease, chronic respiratory disease, diabetes, cancer) are at risk to develop serious illness. On March 11, 2020, the WHO declared it a pandemic.10 Currently, as of April 2020, there have been over 2,300,000 confirmed cases around the globe, including over 150,000 deaths.11
The virus is highly contagious as the reproductive number (R0), which represents the number of secondary infections resulting from an infected individual, is reported to be 2.6.12 Globally, healthcare systems across the world have been faced with unique challenges for controlling the spread of infection. To limit the spread of infection and to preserve limited health care resources, elective surgeries have been canceled or postponed, including pain procedures.13 This has negatively impacted the care of headache patients with complex needs and lead to the interruption of injection of onabotulinumtoxinA for chronic migraine prevention.
Lack of adequate prophylactic therapy may result in frequent ER visits and hospitalizations. Therefore, stable and effective therapy is warranted to improve patients’ quality of life and minimize the utilization of healthcare resources. Although most of the routine visits could be performed using various telemedicine platforms or over the phone, the injections of onabotulinumtoxinA requires in-person procedure visit. OnabotulinumtoxinA injection is an established standard of care prophylactic treatment modality that requires injections to be repeated every 90 days and ideally should not be interrupted.14,15
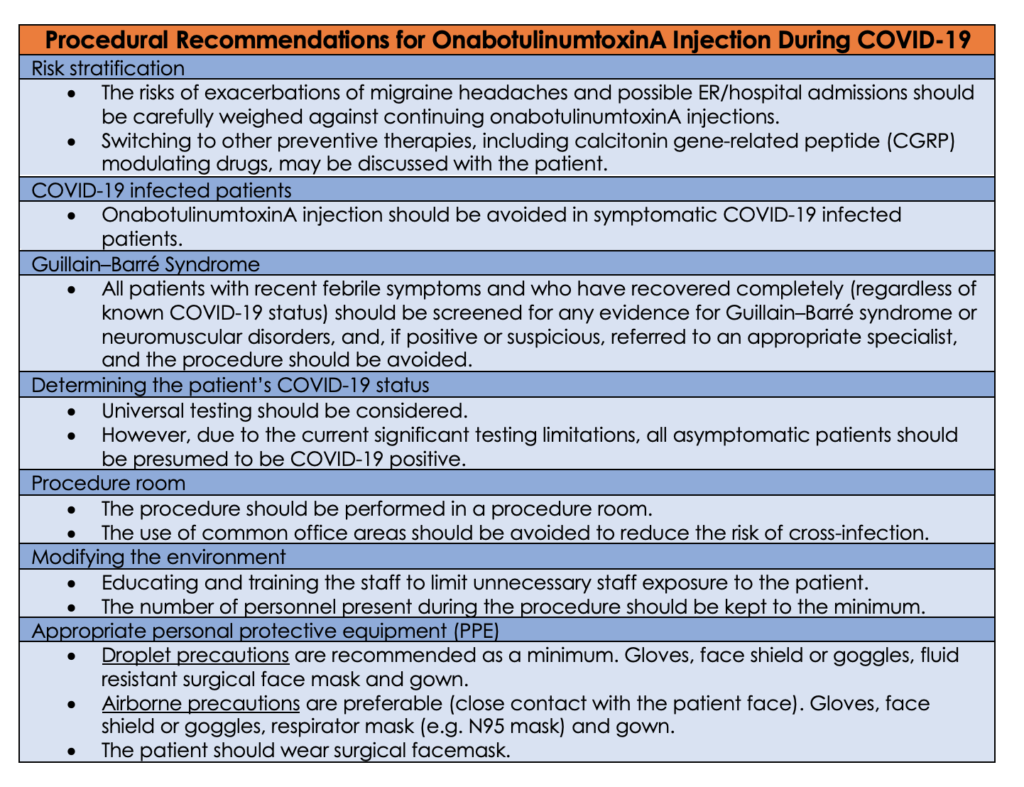
There are special considerations for using onabotulinumtoxinA during COVID-19 pandemic. SARS-CoV-2 presentation varies dramatically, but usually, patients present with fever and cough. There are asymptomatic and there are severe cases. Several cases of new-onset Guillain–Barré syndrome have also been described in patients recently diagnosed with SARS-CoV.16, 17, 18 Guillain–Barré syndrome is a contraindication for onabotulinumtoxinA injection, and therefore, risks and benefits, and alternative treatment options should be carefully weighed and discussed with the patient.19, 20, 21
The Challenges in Identifying the Patient’s COVID-19 Status
Experts advocate for screening all surgical patients to determine their COVID-19 status (e.g. COVID-19 positive, suspected positive (under investigation).22 However, testing capabilities are not widely available yet. If the community spread of COVID-19 infection is significant and in the absence of universal testing, all asymptomatic patients should be presumed to be COVID-19 positive.23 On the other hand, if the community spread is low and a patient is asymptomatic or if tested COVID-19 negative, then the procedure may be performed following usual established guidelines. However; based on recent evidence, The CDC updated their interim guidance on April 12, 2020 This Interim Guidance was updated and archived on April 12, 2020 to reflect new recommendations for community-related exposure to COVID-19, which changed the period of exposure risk from “onset of symptoms” to “48 hours before symptom onset”. 24
Approximately 80% of infected individuals present with no or mild symptoms.25 Accordingly, clinical screening to identify infected patients is not reliable. This emphasizes the need for universal testing. To complicate the challenging clinical situation even further, there is significant variability in the accuracy of different available diagnostic testing modalities with high false-negative rates.26 Hight index of suspension should be exercised and maintain safety precautions. The infection attack rate is estimated to be 50–80% of the population, so all patients should be presumed to have COVID-19 positive.27
Personal Protective Equipment (PPE)
Personal protective equipment is usually classified into three categories: contact precautions, droplet precautions and airborne precautions.28 Generally, appropriate level of PPE is determined by the medical procedure and the proximity of healthcare provider to the patient.
Botox procedures are not considered aerosol-generating procedure (AGP), and therefore droplets precautions are recommended as a minimum.29 However, airborne precautions are preferable as the physician is in remarkable proximity to the patient’s face (unexpected coughing or sneezing). The patient should wear surgical facemask to restrict droplet spread and prevent transmission of COVID-19.30
References
- Burch R, Rizzoli P, Loder E. The Prevalence and Impact of Migraine and Severe Headache in the United States: Figures and Trends From Government Health Studies. Headache. 2018 Apr;58(4):496-505. Pubmed CrossRef
- Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007 Jan 30;68(5):343-349. Pubmed CrossRef
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017 Sep 16;390(10100):1211-1259. Pubmed CrossRef
- World Health Organization. Headache disorders. who.int/mediacentre/factsheets/fs277/en/
- Buse DC, Scher AI, Dodick DW, Reed ML, et al. Impact of Migraine on the Family: Perspectives of People With Migraine and Their Spouse/Domestic Partner in the CaMEO Study. Mayo Clin Proc. 2016;91:596-611. Pubmed CrossRef
- Stewart WF, Wood GC, Manack A, et al. Employment and work impact of chronic migraine and episodic migraine. J Occup Environ Med. 2010 Jan;52(1):8-14. Pubmed CrossRef
- The American Headache Society Position Statement On Integrating New Migraine Treatments Into Clinical Practice. Headache. 2019;59:1-18. Pubmed CrossRef
- Hepp Z, Dodick DW, Varon SF, Chia J, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: A retrospective claims analysis. Cephalalgia. 2017;37:470-485. Pubmed CrossRef
- Hepp Z, Dodick DW, Varon SF, Gillard P, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35:478-488. Pubmed CrossRef
- WHO Director-General’s opening remarks at the media briefing on COVID-19. World Health Organization. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020. Published Mar 11, 2020. Accessed Apr 20, 2020.
- WHO COVID-19 Dashboard. World Health Organization (WHO). https://covid19.who.int/. Accessed Apr 20, 2020.
- Imai N, Cori A, Dorigatti I, et al. Report 3: Transmissibility of 2019-nCoV. Imperial College London. Jan 25, 2020. PDF CrossRef
- Shanthanna H, Strand NH, Provenzano DA, et al. Caring for patients with pain during the COVID-19 pandemic: Consensus recommendations from an international expert panel. Anaesthesia. 2020 Apr 7. PubMed CrossRef
- Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010 Jul;30(7):793–803. PubMed CrossRef
- Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018 Feb 5;19(1):13. PubMed CrossRef
- Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17. PubMed CrossRef
- Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020 Apr 15. PubMed CrossRef
- Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. PubMed CrossRef
- Omprakash Hm, Rajendran Sc. Botulinum Toxin Deaths: What is the Fact?. J Cutan Aesthet Surg. 2008 Jul;1(2):95–97. PubMed CrossRef
- Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55(1):8–14. PubMed CrossRef
- BOTOX (OnabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103000s5302lbl.pdf. Published 1989. Revised Apr 2017. Accessed Apr 21, 2020.
- Wong J, Goh QY, Tan Z, et al. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth. 2020 Mar 11. PubMed CrossRef
- Landau R, Bernstein K, Mhyre J. Lessons learned from first COVID-19 cases in the United States. Anesth Analg. 2020 Mar 31. PubMed CrossRef
- Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Published Apr 15, 2020. Accessed Apr 21, 2020.
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19). Chinese Journal of Epidemiology. 2020 Feb 17;41(2):145-151. PubMed CrossRef
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 Mar 11. PubMed CrossRef
- Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020 Mar 30. pii: S1473-3099(20)30243-7. PubMed CrossRef
- Cook TM. Personal protective equipment during the COVID-19 pandemic – a narrative review. Anaesthesia. 2020 Apr 4. PubMed CrossRef
- Updated guidance on Personal Protective Equipment (PPE) for clinicians. Association of Anaesthetists (AAGBI). https://icmanaesthesiacovid-19.org/personal-protective-equipment-ppe-for-clinicians. Published Apr 11, 2020. Accessed April 21, 2020.
- Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19). World Health Organization (WHO). https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf. Published Feb 27, 2020. Accessed Apr 21, 2020.
Disclosures
Dr. Samer Narouze is the current EIC for Annals of Headache Medicine. Dr. Dmitri Souza was the acting EIC handling this manuscript.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: Samer Narouze MD is the Chairman of the Board and Founder of the American Interventional Headache Society (AIHS) and Alexander Feoktistov, MD, PhD is the President of AIHS.
