Author: Egilius L.H. Spierings, MD, PhD 1
Author Affiliation:
1 Greater Boston Headache Center and MedVadis Research, Boston Advanced Medicine, Waltham, Massachusetts
Competing Interests: The author/s declare no competing interests.
Issue: 12.02
DOI: 10.30756/ahmj.2024.12.02
Received: July 17, 2024
Accepted: Aug 15, 2024
Published: Aug 27, 2024
Recommended Citation: Spierings ELH. The Efficacy Of Continuous Non-Inhaled Intranasal Carbon Dioxide (CO2) In The Abortive Treatment Of Migraine: Results From Three Randomized, Double-Blinded, Placebo-Controlled Trials. Ann Head Med. 2024;12:02. DOI: 10.30756/ahmj.2024.12.02
Objective: Determining the efficacy, tolerability, and safety of continuous non-inhaled intranasal carbon dioxide (CO2) in the acute or abortive treatment of migraine.
Background: Carbon dioxide gas infused in the nose and sinuses potentially blocks trigeminal nerve activation and subsequent neuropeptide release, probably through inhibition of voltage-gated channels. The pathogenesis of the migraine headache involves activation of the trigeminocervical system, which establishes the nociceptive innervation of the head through the trigeminal and occipital nerves.
Methods: In three randomized, double-blinded, placebo-controlled trials, we randomized 350 subjects with migraine as defined by the International Headache Society (intent-to-treat population). We determined tolerability and safety in the 317 subjects who we exposed to treatment up to 24 hours post-dose (modified intent-to-treat population). We determined efficacy in the modified intent-to-treat and per-protocol populations at 15 minutes, half, one, two, and twenty-four hours post-dose in terms of pain freedom, freedom from headache-associated symptoms, and headache relief, treating migraine headaches of mild, moderate, or severe intensity.
Results: We did not observe clinically significant systemic effects, either on blood carbon dioxide pressure or on the cardiovascular or respiratory system. No serious or clinically significant adverse events occurred and those reported were predominantly local, transient, and short-lived. Headache intensity strongly and in a negative way determined the efficacy of treatment with carbon dioxide but not with placebo, and even when placebo consisted of no gas, blinding seemed adequate.
Conclusions: Continuous non-inhaled intranasal carbon dioxide seems safe, relatively well tolerated, and moderately effective for the acute or abortive treatment of migraine.
Introduction
Migraine is a genetically determined and inherited chronic painful condition of the head, characterized by recurrent intense headaches. Its pain mechanisms likely include arterial vasodilation and perivascular inflammation 1, but central disinhibition of nociceptive transmission may play a role as well 2, 3. The vasodilation and inflammation may result, at least partially, from neurogenic inflammation due to the activation of cranial nociceptive nerve fibers 4. Neuropeptides likely mediate this inflammation, especially the potent vasodilator, calcitonin gene-related peptide or CGRP. The effectiveness of the recently approved medications for abortive and preventive migraine treatment targeting the CGRP pathway support the involvement of this neuropeptide 5.
The trigeminocervical system establishes the nociceptive innervation of the head through the trigeminal and occipital nerves. The convergence of those two nerve systems materializes in the trigeminocervical complex, located in the caudal brainstem and superior cervical spinal cord. There, second-order neurons relay the nociceptive information from the cranial peripheral nervous system to the central nervous system, under tonic inhibitory control of the serotoninergic raphé system 2.
Non-invasive access to the trigeminocervical nociceptive system is feasible only through the nose-sinus or nasosinal system, innervated by the ophthalmic (V1) and maxillary branches (V2) of the trigeminal nerve. Numbing of those branches may contribute to alleviating migraine headaches abortively, as suggested by the effectiveness of local anesthetic nasal sprays (5 and 15 minutes 6) and sphenopalatine ganglion blocks (15 and 30 minutes and 2 hours7; 24 hours 8). The sphenopalatine ganglion is a relatively large parasympathetic structure that lies in the nose in close proximity to the maxillary nerve (V2). The same may be true for anesthetic blocks of occipital nerves, but only abortively (one week 9) and not preventively (four weeks 10).
An alternative to local anesthetic nasal sprays or sphenopalatine ganglion blocks is the employment of carbon dioxide (CO2) infused in the nose and sinuses. As a gas, carbon dioxide rapidly penetrates the tissues, enzymatically reacting with water to form carbonic acid (carbonic anhydrase; H2CO3), which quickly dissociates into bicarbonate (HCO3–) and a proton (H+). Neutralization of the extracellularly formed protons leaves the intracellularly formed ones to block nerve activation and subsequent neuropeptide release, probably through inhibition of voltage-gated channels.
Accordingly, under isohydric conditions, that is, in a buffered medium, which probably applies to the nasosinal mucosa because of its rich vascularization, Vause et al.11 demonstrated that carbon dioxide gas represses CGRP release by cultured trigeminal ganglion neurons in response to potassium-induced depolarization. Under those circumstances, carbon dioxide decreases intracellular pH and suppresses the expected increase in intracellular calcium from the potassium-induced depolarization. In rat, Tzabazis et al. 12 demonstrated carbon dioxide insufflation of the nose to increase head withdrawal latency to heat-lamp stimulation of the cheek, suggesting a trigeminal anti-nociceptive or analgesic effect.
Here, we report on the efficacy of continuous non-inhaled intranasal carbon dioxide in the abortive treatment of migraine based on three randomized, double-blinded, placebo-controlled trials. We infused the gas continuously through one nostril while the patient breathed through the mouth, so as not to inhale the carbon dioxide. Capnia Incorporated conducted the trials under the Investigational New Drug (IND) application number 67,485 (United States Food and Drug Administration; US-FDA); they were conducted prior to the mandatory registration on clinicaltrials.gov.
Based on the results, the company had planned two additional abortive migraine trials, one treating mild headaches (NCT00690716; 2012) and the other treating moderate or severe headaches (NCT01253915; 2013) in the hope of obtaining regulatory approval in Europe or the United States. Unfortunately, it could not secure funding for those trials; the development program was subsequently abandoned and the company sold with only two of the trials reported as abstracts 13, 14. Renewed interest from a European company in further developing intranasal carbon dioxide for the abortive treatment of migraine led to review of the available data and compilation of the present manuscript.
Trial CH-2000-2: A physiologic study to evaluate nasal carbon dioxide for relief of symptoms in subjects with migraine headache in a randomized, double-blind, controlled study
Approved by local Institutional Review Boards (IRBs), Xihua Sun, MD (San Jose, California) conducted the trial as lead investigator in 2000-2002 with the assistance of Stasha Gominak, MD (Sunnyvale, California) and Sheena Aurora, MD (Seattle, Washington). They screened fifty-four subjects, 18 years or older, with at least a six-month history of migraine with or without aura and, on average, two to eight moderate or severe headaches per month. Exclusion criteria were significant physical or mental illness, substance abuse, prior rhinoplasty, being pregnant or lactating, and fifteen or more headache days per month (“chronic migraine”).
For treatment, the subjects came to the investigative site within six hours of onset of a moderate or severe migraine headache. They received 100% carbon dioxide or 100% nitrogen in a randomized and double-blinded fashion, administered through a nosepiece that fitted into a nostril. They selected the flow rate of the gas themselves as well as the duration of the treatment, based on preference and tolerability, and held their breath during the gas administration. A data acquisition computer continuously recorded flow rate, respiratory rate, and transcutaneous blood carbon dioxide pressure. An automatic sphygmomanometer measured blood pressure and pulse rate and the investigators used a strip chart to obtain electrocardiograms (ECGs). Women of childbearing potential had a negative urine pregnancy test prior to treatment. The investigators collected adverse events (AEs) up to twenty-four hours after treatment.
The sponsor determined the sample size at seventy-six, thirty-eight in each group, based on two-hour headache relief of 80% for carbon dioxide and 44% for nitrogen (a = 0.05; b = 0.1). They defined headache relief as a reduction in headache intensity from moderate or severe to mild or no pain and calculated the probability of differences using the Chi-Square Test.
For logistic reasons, the sponsor terminated the trial after randomization of fifty subjects, twenty-six to carbon dioxide and twenty-four to placebo. Of the subjects in either group, 88% were women; the median age was 44 years in the carbon dioxide group (range: 20-64) and 48 years in the nitrogen group (range: 31-81). Of the subjects in the carbon dioxide group, 54% had moderate headache at the time of treatment and 46% severe headache. Of those in the nitrogen group, 52% had moderate headache at the time of treatment and 48% severe headache. The headaches treated with carbon dioxide had a median duration before treatment of 3.5 hours and those treated with nitrogen of 3.4 hours.
Table 1 presents the exposures in the two treatment groups, that is, double-blinded 100% carbon dioxide and double-blinded 100% nitrogen. The subjects dosed a median of four times over a median time of twenty-five to thirty minutes with a median of 5.2 and 6.3 liters of 100% carbon dioxide and 100% nitrogen, respectively.
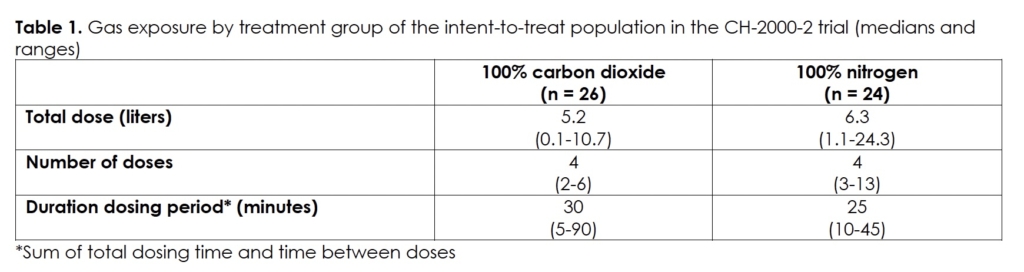
As the trial did not achieve the goal of seventy-six randomized subjects based on the power calculation, we present the efficacy results with this caveat. The tolerability and safety results relate to the fifty randomized subjects (intent-to-treat or ITT population). In those subjects, we did not observe clinically significant changes in either treatment group in heart rate, blood pressure, and blood carbon dioxide pressure, and none of the subjects had a change from baseline in respiratory rate or electrocardiogram.
We did not observe serious or clinically significant adverse events (AEs) in either treatment group. The treatment-emergent adverse events that the subjects reported were transient and short-lived, with the exception of nausea and a sensation of odor in one subject each, which lasted for days. The other adverse events reported by the subjects consisted of nasal irritation and discomfort (burning, tingling, stinging), watery eyes, throat discomfort (burning), unusual or bad taste or smell, and dizziness, lightheadedness, or vertigo.
For the efficacy analysis, we excluded four subjects, three because of protocol violations, all in the nitrogen group, and one because of no post-treatment data (carbon dioxide group). Two subjects, both in the carbon dioxide group, had post-dose data up to one hour post-treatment but not at the two-hour time point. For the efficacy analysis, we imputed the missing two-hour post-dose data based on the one-hour data (last observation carried forward). With these adjustments, the two-hour headache relief was 36% in the carbon dioxide group and 33% in the nitrogen group (p = 0.85).
Trial: CH-2002-1: A study to evaluate nasal carbon dioxide administered using a personal hand-held dispenser for relief of symptoms in subjects with migraine headache in a double-blind, randomized, controlled study
Approved by Health Canada and Trafalgar Ethics Board, Gerald M. Levine, MD (Barrie, Ontario) conducted the trial as lead investigator in Canada in 2002-2003 with the assistance of Wayne Olsheski, MD (Toronto, Ontario); Azim M. Velji, MD (Niagara Falls, Ontario); Fred Fraser, MD (Stoney Creek, Ontario); Brian S. Zidel, MD (Mississauga, Ontario); Paul Ziter, MD (Windsor, Ontario); David J. Broderick, MD (Cobourg, Ontario); Wilson W.S. Leung, MD (Niagara Falls, Ontario); and Esther R. Libman, MD (Thornhill, Ontario).
This was an outpatient trial in which the subjects treated mild, moderate, or severe headache with 100% carbon dioxide at 5 ml/s or 15 ml/s or with placebo. The subjects administered the carbon dioxide intranasally with a hand-held dispenser, consisting of a nosepiece containing a flow-control valve, connected to a small, pressurized cylinder filled with liquid carbon dioxide. They selected the flow rate as low (5 ml/s) or high (15 ml/s) and sealed the nosepiece of the dispenser to a nostril. The subjects randomized to placebo treated with an identical dispenser of which the cylinder did not contain gas and, thus, was empty.
The subjects subsequently allowed the gas to fill the nasosinal system for sixty to ninety seconds, while minimizing breathing. They repeated the dose up to six times with two-to-three-minute intervals, within the two-hour treatment period. The subjects randomized to placebo followed the same procedure, using an identical, hand-held dispenser, which, however, did not contain gas. All could take medication to abort the headache after the two-hour treatment period.
The subjects were 18 years or older and had at least a three-month history of migraine with or without aura. They had a minimum of three and no more than fifteen moderate or severe headaches per month. Exclusion criteria were significant physical or mental illness, substance abuse, taking opioid medications, and being pregnant or lactating. Women of childbearing potential had a negative urine pregnancy test at baseline. The investigators collected adverse events up to twenty-four hours after the initiation of treatment.
The sponsor determined the sample size at 166, eighty-three in each group, based on a two-hour pain-free rate of 35% for carbon dioxide and 15% for placebo (a = 0.05; b = 0.1). They imputed missing data by carrying the last observation forward. They calculated the probability of differences using the Chi-Square Test and determined correlation coefficients employing the Pearson Test.
Of the 152 subjects in the trial, the sponsor randomized seventy-five to carbon dioxide and seventy-seven to placebo (intent-to-treat population). In the carbon dioxide group, eight withdrew consent before treatment and twelve withdrew consent in the placebo group. Therefore, the modified intent-to-treat (m-ITT) population exposed to treatment consisted of sixty-seven subjects in the carbon dioxide group and sixty-five in the placebo group. In the carbon dioxide group, ten subjects did not submit evaluable diaries and in two, the dispenser was defective. In the placebo group, five subjects did not submit evaluable diaries. Therefore, the per-protocol (PP) population consisted of fifty subjects in the carbon dioxide group and sixty in the placebo group.
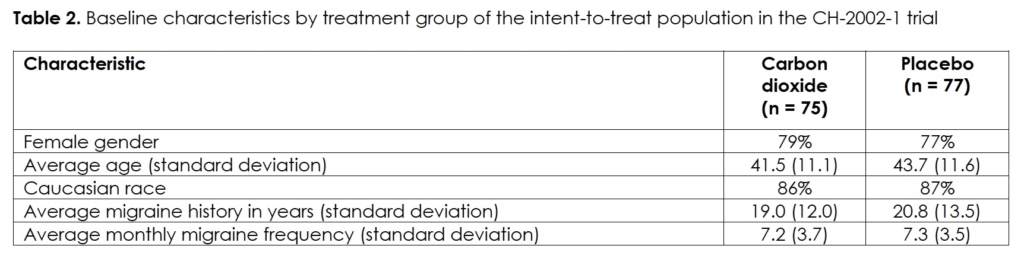
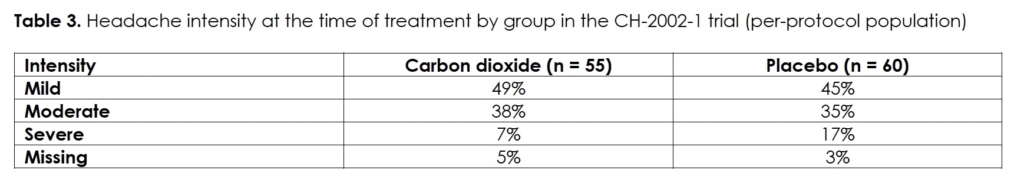
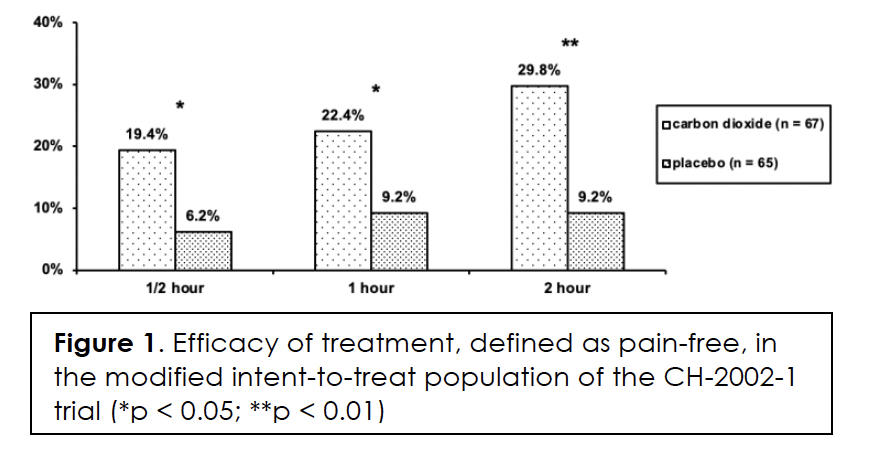
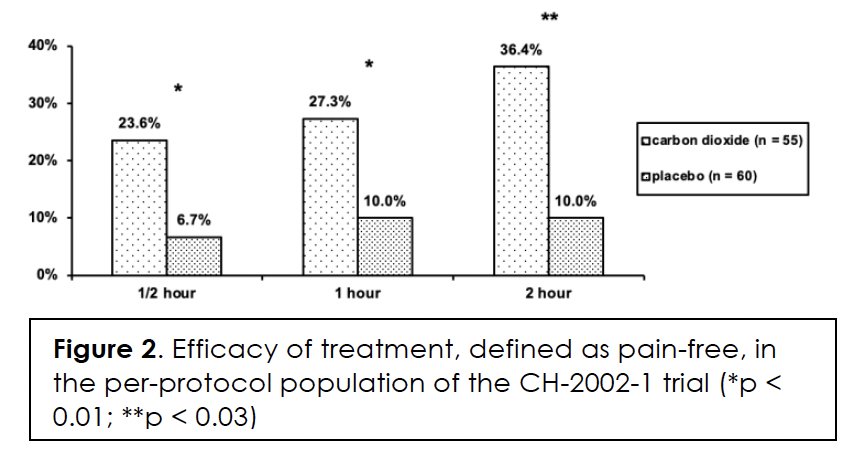
We present the demographics and migraine characteristics of the intent-to-treat population by group in Table 2; there were no statistically significant differences between them. The subjects treated with a medium number of three dosages (range: 1 – 7). Efficacy in terms of pain-free at half, one, and two hours after initiation of treatment, we show in Figures 1&2 for the modified intent-to-treat and per-protocol population, respectively. The differences between the two treatment groups are statistically significant for both populations at every post-dose time point. We present the intensity of the headaches at the time of treatment by group of the per-protocol population in Table 3. The difference in distribution between the two groups was not statistically significant (p>0.46). Efficacy correlated negatively with headache intensity in the carbon dioxide group (r = -0.99; p<0.05) but not in the placebo group (r = -0.67; p>0.5).
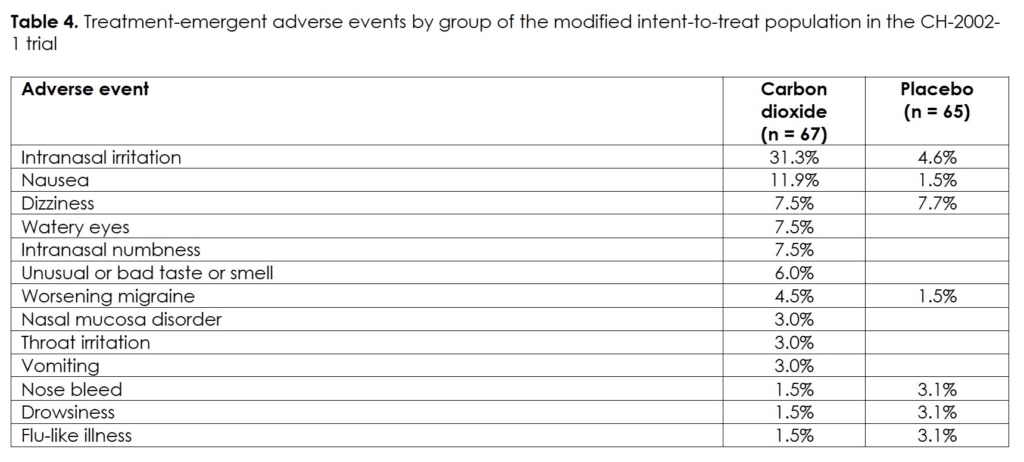
There were no serious or clinically significant adverse events in the intent-to-treat population of the study. Table 4 lists the treatment-emergent adverse events by group with an occurrence of 3% or more; all were transient in nature and resolved without treatment.
Trial CH-2004-3: Multi-center, randomized, double-blind study to evaluate the dose-regimen related efficacy and safety of CAP3 in the treatment of acute migraine (ESCAPE)
Approved by Western Institutional Review Board (WIRB) and local ethics boards as applicable, Roger Cady (Springfield, Missouri) conducted the trial as lead investigator in 2004-2005 with the assistance of James R. Couch, MD (Oklahoma City, Oklahoma); David W. Dodick, MD (Rochester, Minnesota); Frederick G. Freitag, DO (Chicago, Illinois); Jerome Goldstein, MD (San Francisco, California); Jack A. Klapper, MD (Denver, Colorado); Dawn A. Marcus, MD (Pittsburgh, Pennsylvania); Ninan T. Mathew, MD (Houston, Texas); Todd D. Rozen, MD (Ann Arbor, Michigan); Gary E. Ruoff, MD (Kalamazoo, Michigan); Egilius L.H. Spierings, MD, PhD (Wellesley Hills, Massachusetts); and William B. Young, MD (Philadelphia, Pennsylvania).
This was an outpatient trial in which the subjects treated moderate or severe headache. They treated with 100% carbon dioxide at 10 ml/s or with a placebo device that released no gas at the nose. The subjects administered the treatment with a hand-held dispenser, consisting of a nosepiece containing a flow-control valve, connected to a small, pressurized cylinder filled with liquid carbon dioxide. They sealed the nosepiece of the dispenser to a nostril and allowed the gas to fill the nasosinal system for ninety seconds, while minimizing breathing. They repeated the dose up to six times with three-to-five minute intervals, within the two-hour treatment period.
The subjects randomized to the placebo group followed the same procedure, using an identical, hand-held dispenser. This dispenser, however, contained a diverter that blocked the flow of carbon dioxide at the nozzle, causing the gas to leak imperceptibly from the bottom of the device. All could take medication to abort the headache after the two-hour treatment period.
The subjects were 18 years to 75 years of age and had at least a twelve-month history of migraine with or without aura. They had two to eight moderate or severe headaches per month with at least 48 hours between them and six or less non-migraine headaches. Exclusion criteria were significant physical or mental illness, substance abuse, and being pregnant or lactating. Women of childbearing potential had a negative urine pregnancy test at baseline. The investigators collected adverse events up to twenty-four hours after the initiation of treatment.
The sponsor determined the sample size at 153 subjects randomized two to one, based on a two-hour pain-free rate of 35% for carbon dioxide and 10% for placebo (a = 0.05; b = 0.05). They imputed missing data by carrying the last observation forward. They calculated the probability of differences using the Chi-Square Test or the Fisher Exact Test.
Of the 148 subjects in the trial, the sponsor randomized ninety-nine to the carbon dioxide group and forty-nine to the placebo group (intent-to-treat population). In the carbon dioxide group, one subject withdrew consent before treatment, five did not have a qualifying migraine headache within the sixty-day treatment period, and three did not treat a qualifying migraine with study medication. In the placebo group, one subject withdrew consent before treatment, one did not have a qualifying migraine headache within the sixty-day treatment period, and two did not treat a qualifying migraine with study medication. Therefore, the modified intent-to-treat population exposed to treatment consisted of ninety subjects in the carbon dioxide group and forty-five in the placebo group. In the carbon dioxide group, one subject was lost to follow up and, therefore, the per-protocol population consisted of eighty-nine subjects in the carbon dioxide group and forty-five in the placebo group.
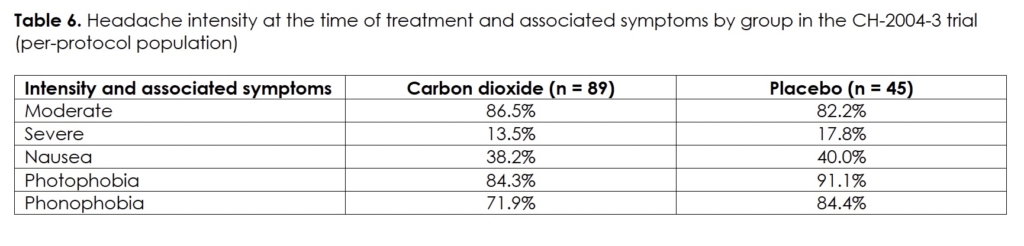
We present the demographics and migraine characteristics of the intent-to-treat population in Table 5. We show the intensity of the headaches at the time of treatment and the frequency of associated symptoms of the per-protocol population in Table 6. We present both by group and there were no statistically significant differences between them. The subjects administered a median of five dosages per treatment in the carbon dioxide group (range: 1 – 7) and six dosages in the placebo group (range: 1 – 7). The median gas exposure in the carbon dioxide group was 4.5 liters (range: 0.9 – 6.3).
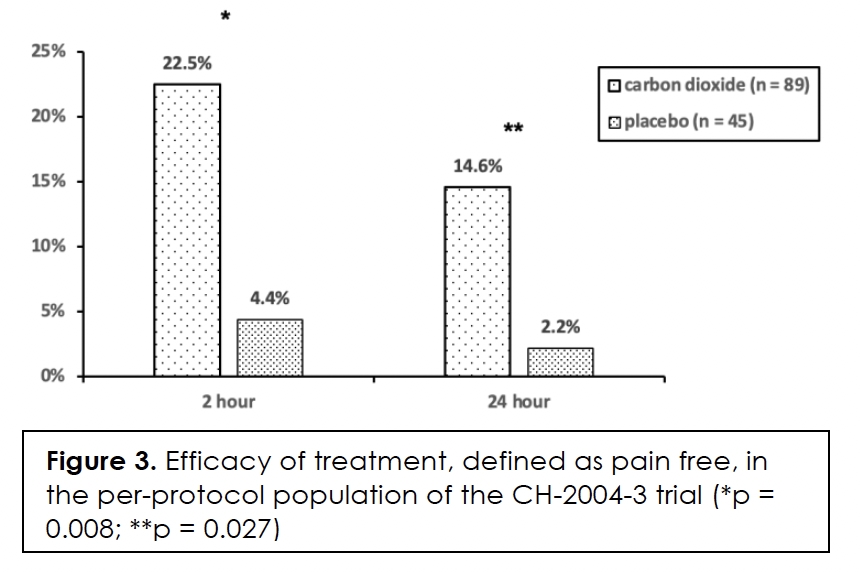
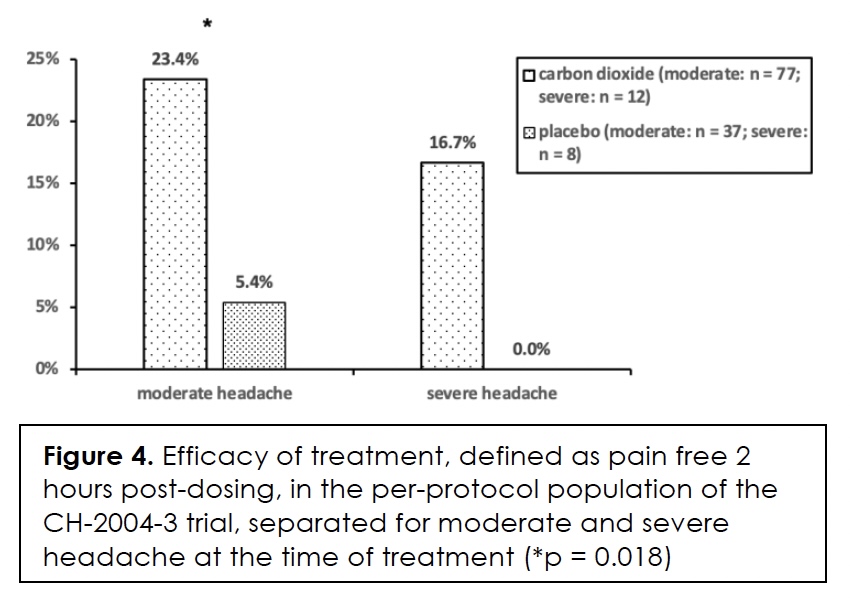
Efficacy in terms of pain-free at two and twenty-four hours after initiation of treatment, we show in Figure 3 for the per-protocol population. The differences between the two groups are statistically significant at both time points. We present efficacy in terms of pain-free at two hours in relation to headache intensity at the time of treatment in Figure 4. The difference between the two groups is only statistically significant for moderate headache (p = 0.018). The effect size for severe headache is relatively large at 16.7% but the number of subjects in the groups is small.
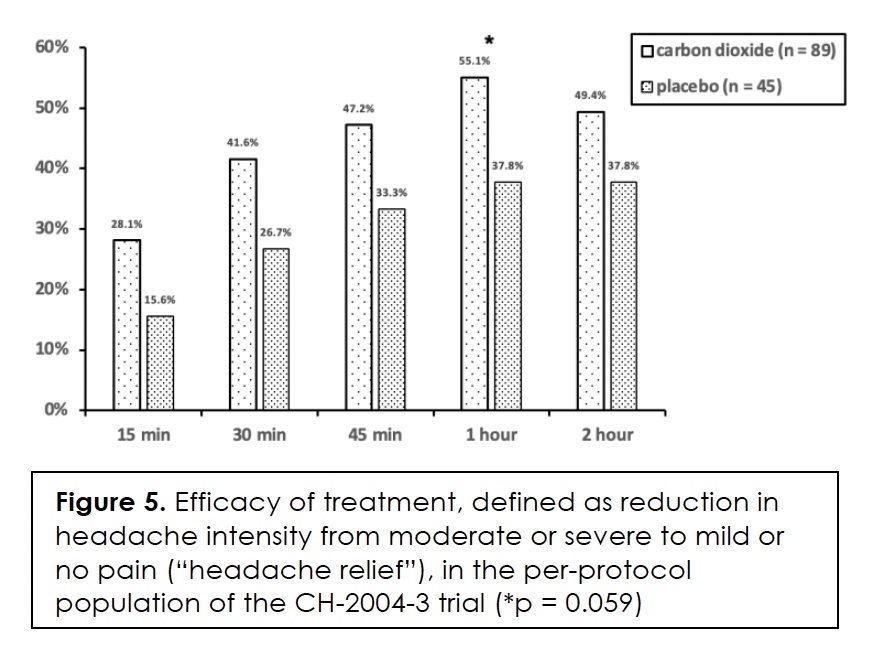
In Figure 5, we present the efficacy of treatment in terms of headache relief, that is, a reduction in headache intensity from moderate or severe to mild or no pain, over the two-hour, post-dose time period. The numerical differences between the two groups ranged from 11.6% to 17.3% in favor of carbon dioxide; it was almost statistically significant (p = 0.059) at one-hour post-dose when the effect size was 17.3%.
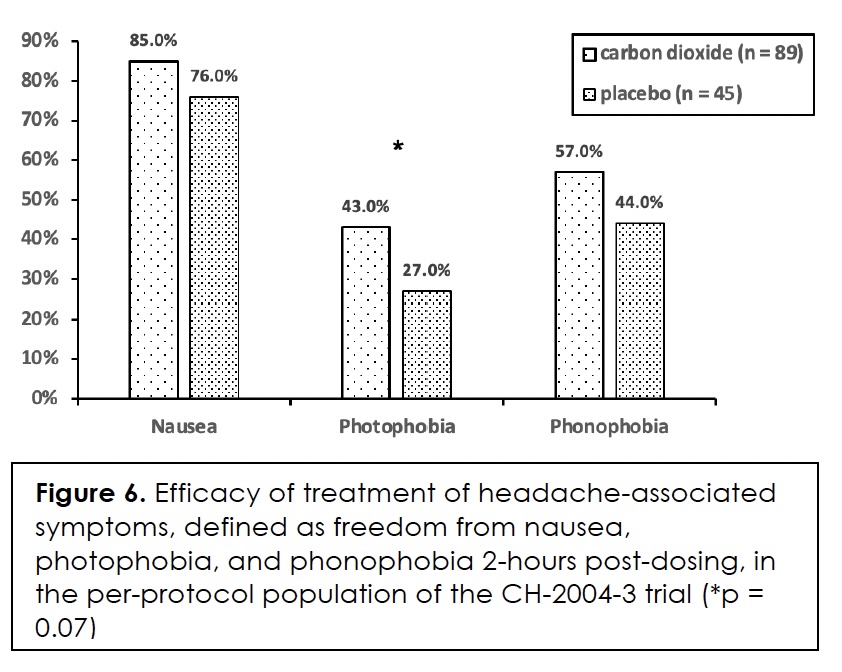
The efficacy of treatment in terms of freedom from headache-associated symptoms, that is, nausea, photophobia, and phonophobia, we show in Figure 6. The differences between the two groups did not reach statistical significance for any of the symptoms but came close for photophobia (p = 0.07).
In terms of serious adverse events or deaths, there were none in the trial. Treatment-emergent adverse events that occurred in 3% or more were nasal congestion (3.4% in the carbon dioxide group and 0% in the placebo group), pharyngolaryngeal pain (3.4% and 0%, respectively), and dizziness (6.7% and 4.4%, respectively).
At the end of the trial, the investigators asked the subjects, which study medication they believed to have received: drug, placebo, or not sure. Of the subjects in the carbon dioxide group, 20.2% indicated “not sure” and this was the case with 35.5% in the placebo group. Of the remaining subjects, the treatment outcome very much determined the response. Of the subjects rendered pain-free, 100% in the carbon dioxide group and 80% in the placebo group indicated “drug”; these numbers were 51% and 30%, respectively, in those who did not obtain this particular benefit.

Discussion
Apart from the abortive treatment of migraine, Capnia studied the efficacy, safety, and tolerability of non-inhaled intranasal carbon dioxide for the treatment of seasonal allergic rhinitis 15. This clinical development program was successful and resulted in a nonprescription product for the symptomatic relief of allergic rhinitis in the United Kingdom and Ireland under the brand name Serenz®. Unfortunately, the sponsor never completed the development program for migraine, predominantly because of funding issues. The purpose of reporting the relevant clinical trials here is because of the author’s belief that 1. We should report all clinical trials in full for the benefit of society, whether meeting primary endpoint or not; and 2. Continuous non-inhaled intranasal carbon dioxide remains a potential option for the abortive treatment of migraine, as the results reported here suggest, although further clinical trials are required.
The three randomized, double-blinded, placebo-controlled trials reported herein constitute the first, or phase I-II, part of the clinical development program of continuous non-inhaled intranasal carbon dioxide for the acute or abortive treatment of migraine. The first trial, CH-2000-2, focused on safety and tolerability, not in healthy volunteers as is usually the case but in migraineurs to also obtain an assessment of its potential efficacy. The sponsor conducted the trial at three investigative sites only, which led to protracted enrollment that ultimately resulted in its premature termination. The enrollment goal was seventy-six subjects randomized of which the trial, with fifty subjects randomized, only realized 66%.
In the trial, the investigators not only assessed tolerability through adverse event collection but also measured blood pressure, pulse rate, respiratory rate, and transcutaneous carbon dioxide pressure and obtained electrocardiograms as well. They observed no changes in any of those safety parameters with either 100% carbon dioxide or 100% nitrogen, with a median of 5-6 liters administered to each subject over a median time of 25-30 minutes. The adverse events that the subjects reported were transient and short-lived, with the exception of nausea and a sensation of odor in one subject each, which lasted for days. The reported adverse events consisted of nasal irritation and discomfort (burning, tingling, stinging), watery eyes, throat discomfort (burning), unusual or bad taste or smell, and dizziness, lightheadedness, or vertigo.
The subjects treated moderate or severe headache in approximately a fifty-fifty distribution, and the sponsor determined the efficacy in terms of two-hour headache relief, that is, a reduction in headache intensity from moderate or severe to mild or no pain. A third of the subjects experienced this benefit with either treatment, and the difference between the two groups was not statistically significant. The caveat with the observed efficacy is that the trial did not meet the required randomized numbers as per the power calculation.
The second trial, CH-2002-1, differed from the first one in two aspects: 1. It was an outpatient trial in which the subjects treated at home (or at work) as opposed to at the investigative site; and 2. It also allowed them to treat a migraine headache at mild headache intensity as opposed to at moderate or severe intensity only. The subjects treated with 100% carbon dioxide at a self-selected rate of 5 ml/s or 15 ml/s or no gas (placebo). The trial allowed them to repeat the dose up to six times with two-to-three-minute intervals, within the two-hour treatment period. The subjects treated with a medium number of three dosages (range: 1 – 7). The nine investigators completed the enrollment close to the randomized number required as per the power calculation (92%).
In terms of safety and tolerability, there were no serious or clinically significant adverse events in the trial. In terms of treatment-emergent adverse events, intranasal irritation was the most common and occurred in almost one-third of the subjects receiving carbon dioxide, followed by nausea in over 10%, compared to less than 5% with placebo. Dizziness occurred to the same extent in both treatment groups but watery eyes, intranasal numbness, and unusual or bad taste or smell occurred in 6.0% to 7.5% of the carbon dioxide group and none in the placebo group. However, all adverse events were transient in nature and resolved spontaneously, that is, without treatment.
With the subjects also allowed to treat migraine headaches at mild headache intensity, the sponsor determined efficacy in terms of pain-free and did so at half, one, and two hours after initiation of treatment. Almost half of the subjects treated their migraine headaches at mild headache intensity, over a third at moderate intensity, and around 10% at severe intensity. At all three time points, the efficacy was statistically significantly better in the group treated with carbon dioxide than in that treated with placebo, both for the modified intent-to-treat and the per-protocol population. It correlated negatively with headache intensity at the time of treatment in the carbon dioxide group but not in the placebo group, suggesting a biological effect because we would indeed expect the benefit to correlate with headache intensity.
The third trial, CH-2004-3, was essentially a repeat of the second one but treating migraine headaches at moderate or severe headache intensity only as per the requirement of the US-FDA. The subjects treated with 100% carbon dioxide at 10 ml/s or with a placebo that released no gas at the nose. They treated with a median of five or six dosages (range: 1 – 7) and a median gas exposure of 4.5 liters (range: 0.9 – 6.3)(carbon dioxide group). The twelve investigators completed the enrollment close to the randomized number required as per the power calculation (97%).
In terms of serious adverse events or deaths, there were none in the trial. Treatment-emergent adverse events in the carbon dioxide group were nasal congestion (3.4%) and pharyngolaryngeal pain (3.4%), compared to none in the placebo group; dizziness occurred almost equally in both groups. The sponsor determined efficacy in terms of 1. Pain-free at two and twenty-four hours after initiation of treatment; 2. Freedom from headache-associated symptoms, that is, nausea, photophobia, and phonophobia, at two hours and; 3. Headache relief, that is, a reduction in headache intensity from moderate or severe to mild or no pain, from fifteen minutes to two hours. Of these three endpoints, of which the two-hour pain freedom was the primary, only the first one, that is, pain-free at two and twenty-four hours, was statistically significant between the two groups in favor of carbon dioxide, at both time points and for the per-protocol population.
In this trial, the sponsor also addressed the issue of blinding, especially because in the second and third trials the placebo consisted of no gas and the intranasal administration of carbon dioxide is not without tolerability issues, although less so in this trial than in the second one. For this purpose, the investigators asked the subjects at the end of the trial, which study medication they believed to have received: drug, placebo, or not sure. With a positive treatment outcome, the response was “drug” in 100% in the carbon dioxide group and 80% in the placebo group; with a negative treatment outcome, the “drug” response was 50% and 30%, respectively. Hence, the response seems driven more by treatment outcome than by group assignment, which suggests adequate blinding.
Conclusion
The three randomized, double-blinded, placebo-controlled trials reported herein suggest continuous non-inhaled intranasal carbon dioxide to be safe, relatively well tolerated, and moderately effective for the acute or abortive treatment of migraine, warranting further clinical development.
Acknowledgements
I would like to thank Gerard Pereira, PhD, MBA, for kindly providing me with the data and the clinical trial reports and Victor Wang, PhD, MD, MBA, for critically reviewing the manuscript.
References
- Spierings ELH. The aura-headache connection in migraine: a historical analysis. Arch Neurol. May 2004;61(5):794-9. PubMed PMID: 15148162. doi:10.1001/archneur.61.5.794
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. Nov 1978;4(5):451-62. PubMed PMID: 216303. doi:10.1002/ana.410040511
- Spierings ELH. Endogenous pain control mechanisms. Ann Neurol. Jul 1979;6(1):89. PubMed PMID: 507772. doi:10.1002/ana.410060131
- Spierings ELH, Sanchez del Rio M, editors. Migraine: A Neuroinflammatory Disease? 1 ed. Birkhäuser Basel; 2002. https://doi.org/10.1007/978-3-0348-8131-9
- Spierings ELH, Ko M. New entries into the migraine market. J Neurol Neurosurg. Apr 2023;18(1):555980. doi: 10.19080/OAJNN.2023.18.555980
- Chi PW, Hsieh KY, Chen KY, et al. Intranasal lidocaine for acute migraine: A meta-analysis of randomized controlled trials. PLoS One. 2019;14(10):e0224285. PubMed PMID: 31644605; PubMed Central PMCID: PMCPMC6808552. doi:10.1371/journal.pone.0224285
- Cady R, Saper J, Dexter K, Manley HR. A double-blind, placebo-controlled study of repetitive transnasal sphenopalatine ganglion blockade with tx360((R)) as acute treatment for chronic migraine. Headache. Jan 2015;55(1):101-16. PubMed PMID: 25338927; PubMed Central PMCID: PMC4320756. doi:10.1111/head.12458
- Schaffer JT, Hunter BR, Ball KM, Weaver CS. Noninvasive sphenopalatine ganglion block for acute headache in the emergency department: a randomized placebo-controlled trial. Ann Emerg Med. May 2015;65(5):503-10. PubMed PMID: 25577713. doi:10.1016/j.annemergmed.2014.12.012
- Cuadrado ML, Aledo-Serrano A, Navarro P, et al. Short-term effects of greater occipital nerve blocks in chronic migraine: A double-blind, randomised, placebo-controlled clinical trial. Cephalalgia. Aug 2017;37(9):864-872. PubMed PMID: 27296456. doi:10.1177/0333102416655159
- Dilli E, Halker R, Vargas B, et al. Occipital nerve block for the short-term preventive treatment of migraine: A randomized, double-blinded, placebo-controlled study. Cephalalgia. Oct 2015;35(11):959-68. PubMed PMID: 25505035. doi:10.1177/0333102414561872
- Vause C, Bowen E, Spierings ELH, Durham P. Effect of carbon dioxide on calcitonin gene-related peptide secretion from trigeminal neurons. Headache. Nov-Dec 2007;47(10):1385-97. PubMed PMID: 18052948; PubMed Central PMCID: PMCPMC3138149. doi:10.1111/j.1526-4610.2007.00850.x
- Tzabazis AZ, Niv SH, Manering NA, et al. Trigeminal antihyperalgesic effect of intranasal carbon dioxide. Life Sci. Jul 3 2010;87(1-2):36-41. PubMed PMID: 20561904; PubMed Central PMCID: PMCPMC2900516. doi:10.1016/j.lfs.2010.05.013
- Spierings ELH. Abortive treatment of migraine headache with non-inhaled, intranasal carbon dioxide: A randomized, double-blind, placebo-controlled, parallel-group study. Headache The Journal of Head and Face Pain. Jun 2005;45(6):809.
- Spierings ELH. Non-inhaled, intranasal carbon dioxide for the abortive treatment of migraine headache: Efficacy, tolerability, and safety. Ann Neurol. 2005;58(S17)(59).
- Casale TB, Romero FA, Spierings ELH. Intranasal noninhaled carbon dioxide for the symptomatic treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. Jan 2008;121(1):105-9. PubMed PMID: 18028997. doi:10.1016/j.jaci.2007.08.056
Declarations/Disclosures
Consent/Permission/Ethics Approval: Not indicated based on the type of manuscript
Funding/Conflicts of interest: In compliance with the ICMJE uniform disclosure form, author declares the following:
Payment/services info: Author has declared that no financial support was received from any organization for the submitted work.
Financial relationships: Author has declared that he has no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. From 2004 until 2007, the author was the (part-time) chief medical officer (CMO) of Capnia Incorporated, a company no longer in existence. He wrote the present manuscript without financial support.
Other relationships: Author has declared that there are no other relationships or activities that could appear to have influenced the submitted work.
